
Chemistry
Paper 1 | Objectives | 49 Questions
JAMB Exam
Year: 2008
Level: SHS
Time:
Type: Question Paper
Answers provided
FREE
No description provided
Feedbacks
This paper is yet to be rated

Paper 1 | Objectives | 49 Questions
JAMB Exam
Year: 2008
Level: SHS
Time:
Type: Question Paper
Answers provided
No description provided
This paper is yet to be rated
These are the best study techniques and methods that get higher grades in any school tests or exams.
Try studying past questions since it's a sure way to better grades in any subject at school and beyond.
Make sure you study hard but not into the late-night hours to give your body the enough rest you need.
| # | Question | Ans |
|---|---|---|
| 1. |
Chlorophyll obtained from given leaves of plant can be shown to be composed of more than one coloured component by the technique of A. crystallization B. hydrolysis C. chromatography D. sublimation |
C |
| 2. |
In countries where the temperature falls below 273K, salt is always sprinkled on the icy road in order to A. lower the melting point of the ice B. increase the density of the ice C. make the ice impure D. raise the melting point of the ice |
A |
| 3. |
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) A. 0.40g B. 0.80g C. 4.00g D. 8.00g
Show Content
Detailed Solution2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)Relative molecular mass of NaOH = 23 + 16 + 1 = 40smol-1 2 * 23g of sodium react with water to produce 2 * 40g of NaOH ∴ 2.3g of sodium will react with water to produce (2.3 * 40 * 2)/(23 * 2) = 4.00g |
|
| 4. |
16.8 g of sodium hydrogen trioxocarbonate (IV) is completely decomposed by heat. Calculate the volume of carbon(IV) oxide given off s.t.p A. 22.40 dm3 B. 11.20 dm3 C. 2.24 dm3 D. 1.12 dm3
Show Content
Detailed Solution2NaHCO3(s) → Na2CO3(s) + H2O(l) + CO2Relative molecular mass of NaHCO3 = 23 +1 + 12 + 16 * 3 = 84smol-1 2 * 84g of NaHCO3 will be liberated 22.4dm3 or CO2 at s.t.p 16.8g of NaHCO3 will libertae (16.8 * 22.4)/(2 * 84) = 2.24 dm3 |
|
| 5. |
300 cm3 of gas has a pressure of 800 mmHg. If the pressure is reduced to 650 mmHg, find its volume A. 243.75 cm3 B. 369.23 cm3 C. 738.46 cm3 D. 1733.36 cm3
Show Content
Detailed SolutionP1V1= P2V2800 * 300 = 650 * V2 V2 = (800 * 300)/650 = 369.23 cm3 |
|
| 6. |
Diffusion is slowest in solid particles than in particles of liquid and gases because A. solid particles have more kinetic energy than the particles of liquid and gases B. solid particles have less kinetic energy than the particles of liqiud and gases C. solid particles have less restriction in their movement than liquid and gas particles D. the particles in solids are far apart and the cohesive forces between them are negligible |
B |
| 7. |
The experiment that showed that atoms have tiny positively charged nucleus was first carried out by A. Moseley B. Rutherford C. Millikan D. Dalton |
B |
| 8. |
The atom of an element X is represented as zyX. The basic chemical properties of X depends on the value of A. Y B. Z C. Y - Z D. Z - Y |
B |
| 9. |
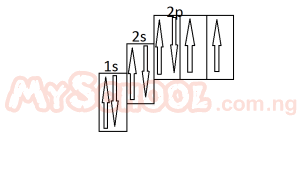 The diagram above represent the electron sub-level for A. carbon B. nitrogen C. oxygen D. flourine |
C |
| 10. |
In the periodic table, the electrical and thermal conductivities are properties of elements that A. decrease across the period and increase down the group B. increase across the period and decrease down the group C. decrease across the period and down the group` D. increase acrosss the period and down the group |
A |
Preview displays only 10 out of the 49 Questions