Year :
2008
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
1 - 10 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 1. |
Chlorophyll obtained from given leaves of plant can be shown to be composed of more than one coloured component by the technique of A. crystallization B. hydrolysis C. chromatography D. sublimation |
C |
| 2. |
In countries where the temperature falls below 273K, salt is always sprinkled on the icy road in order to A. lower the melting point of the ice B. increase the density of the ice C. make the ice impure D. raise the melting point of the ice |
A |
| 3. |
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) A. 0.40g B. 0.80g C. 4.00g D. 8.00g Detailed Solution2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)Relative molecular mass of NaOH = 23 + 16 + 1 = 40smol-1 2 * 23g of sodium react with water to produce 2 * 40g of NaOH ∴ 2.3g of sodium will react with water to produce (2.3 * 40 * 2)/(23 * 2) = 4.00g |
|
| 4. |
16.8 g of sodium hydrogen trioxocarbonate (IV) is completely decomposed by heat. Calculate the volume of carbon(IV) oxide given off s.t.p A. 22.40 dm3 B. 11.20 dm3 C. 2.24 dm3 D. 1.12 dm3 Detailed Solution2NaHCO3(s) → Na2CO3(s) + H2O(l) + CO2Relative molecular mass of NaHCO3 = 23 +1 + 12 + 16 * 3 = 84smol-1 2 * 84g of NaHCO3 will be liberated 22.4dm3 or CO2 at s.t.p 16.8g of NaHCO3 will libertae (16.8 * 22.4)/(2 * 84) = 2.24 dm3 |
|
| 5. |
300 cm3 of gas has a pressure of 800 mmHg. If the pressure is reduced to 650 mmHg, find its volume A. 243.75 cm3 B. 369.23 cm3 C. 738.46 cm3 D. 1733.36 cm3 Detailed SolutionP1V1= P2V2800 * 300 = 650 * V2 V2 = (800 * 300)/650 = 369.23 cm3 |
|
| 6. |
Diffusion is slowest in solid particles than in particles of liquid and gases because A. solid particles have more kinetic energy than the particles of liquid and gases B. solid particles have less kinetic energy than the particles of liqiud and gases C. solid particles have less restriction in their movement than liquid and gas particles D. the particles in solids are far apart and the cohesive forces between them are negligible |
B |
| 7. |
The experiment that showed that atoms have tiny positively charged nucleus was first carried out by A. Moseley B. Rutherford C. Millikan D. Dalton |
B |
| 8. |
The atom of an element X is represented as zyX. The basic chemical properties of X depends on the value of A. Y B. Z C. Y - Z D. Z - Y |
B |
| 9. |
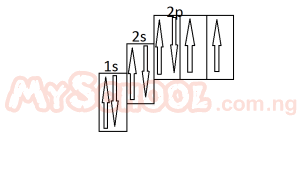 The diagram above represent the electron sub-level for A. carbon B. nitrogen C. oxygen D. flourine |
C |
| 10. |
In the periodic table, the electrical and thermal conductivities are properties of elements that A. decrease across the period and increase down the group B. increase across the period and decrease down the group C. decrease across the period and down the group` D. increase acrosss the period and down the group |
A |
| 1. |
Chlorophyll obtained from given leaves of plant can be shown to be composed of more than one coloured component by the technique of A. crystallization B. hydrolysis C. chromatography D. sublimation |
C |
| 2. |
In countries where the temperature falls below 273K, salt is always sprinkled on the icy road in order to A. lower the melting point of the ice B. increase the density of the ice C. make the ice impure D. raise the melting point of the ice |
A |
| 3. |
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) A. 0.40g B. 0.80g C. 4.00g D. 8.00g Detailed Solution2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)Relative molecular mass of NaOH = 23 + 16 + 1 = 40smol-1 2 * 23g of sodium react with water to produce 2 * 40g of NaOH ∴ 2.3g of sodium will react with water to produce (2.3 * 40 * 2)/(23 * 2) = 4.00g |
|
| 4. |
16.8 g of sodium hydrogen trioxocarbonate (IV) is completely decomposed by heat. Calculate the volume of carbon(IV) oxide given off s.t.p A. 22.40 dm3 B. 11.20 dm3 C. 2.24 dm3 D. 1.12 dm3 Detailed Solution2NaHCO3(s) → Na2CO3(s) + H2O(l) + CO2Relative molecular mass of NaHCO3 = 23 +1 + 12 + 16 * 3 = 84smol-1 2 * 84g of NaHCO3 will be liberated 22.4dm3 or CO2 at s.t.p 16.8g of NaHCO3 will libertae (16.8 * 22.4)/(2 * 84) = 2.24 dm3 |
|
| 5. |
300 cm3 of gas has a pressure of 800 mmHg. If the pressure is reduced to 650 mmHg, find its volume A. 243.75 cm3 B. 369.23 cm3 C. 738.46 cm3 D. 1733.36 cm3 Detailed SolutionP1V1= P2V2800 * 300 = 650 * V2 V2 = (800 * 300)/650 = 369.23 cm3 |
| 6. |
Diffusion is slowest in solid particles than in particles of liquid and gases because A. solid particles have more kinetic energy than the particles of liquid and gases B. solid particles have less kinetic energy than the particles of liqiud and gases C. solid particles have less restriction in their movement than liquid and gas particles D. the particles in solids are far apart and the cohesive forces between them are negligible |
B |
| 7. |
The experiment that showed that atoms have tiny positively charged nucleus was first carried out by A. Moseley B. Rutherford C. Millikan D. Dalton |
B |
| 8. |
The atom of an element X is represented as zyX. The basic chemical properties of X depends on the value of A. Y B. Z C. Y - Z D. Z - Y |
B |
| 9. |
 The diagram above represent the electron sub-level for A. carbon B. nitrogen C. oxygen D. flourine |
C |
| 10. |
In the periodic table, the electrical and thermal conductivities are properties of elements that A. decrease across the period and increase down the group B. increase across the period and decrease down the group C. decrease across the period and down the group` D. increase acrosss the period and down the group |
A |