Year :
2018
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
21 - 30 of 87 Questions
| # | Question | Ans |
|---|---|---|
| 21. |
Which of the following pairs of substances will react further with oxygen to form a higher oxide? A. CO\(_2\) and H\(_2\)O B. NO and H\(_2\)O C. CO and CO\(_2\) D. SO\(_2\) and NO |
|
| 22. |
In the preparation of oxygen by heating KCIO\(_3\) , in the presence of MnO\(_2\) only moderate heat is needed because the catalyst acts by \(_2\) A. lowering the pressure of the reaction B. increasing the surface area of the reaction C. increasing the rate of the reaction D. increasing the energy barrier of the reaction |
|
| 23. |
Methanoic acid mixes with water in all proportions and has about the same boiling point as water. Which of the following methods would you adopt to obtain pure water from a mixture of Sand, water and methanoic acid? A. Extractive distillation B. Filtration followed by distillation C. Neutralization with sodium hydroxide followed by distillation D. Neutralization with sodium hydroxide followed by filtration Detailed SolutionDue to the presence of maximum Azeotrope,we use extractive distilination (with extractive distilation agents like sulfone) used for mixtures with low relative volatility,nearing unity.Azeotrope is a mixture of liquids that has a constant boiling point because the vapour has thesame composition as the mixture. There is an explanation video available below. |
|
| 24. |
A quantity of electricity liberates 3.6g of Silver from its salt. What mass of aluminium Will be liberated from its salt by the same quantity of electricity? [Al = 27, Ag = 108]. A. 2.7g B. 1.2g C. 0.9g D. 0.3g |
|
| 25. |
Suitable reagents for the laboratory preparation of nitrogen are A. sodium dioxonitrate(III) and ammonium chloride B. sodium trioxonitrate(V) and ammonium chloride C. sodium chloride and ammonium trioxonitrate(V) D. sodium chloride and ammonium di-ozonitrate(III) |
|
| 26. |
The number of electrons in the valence shell of an element of atomic number 14 is? A. 1 B. 2 C. 3 D. 4 |
|
| 27. |
What volume of oxygen will remain after reacting 8cm\(^3\) of hydrogen gas with 20cm\(^3\) of oxygen gas A. 10cm\(^3\) B. 12cm\(^3\) C. 14cm\(^3\) D. 16cm\(^3\) |
|
| 28. |
If one of the following oxides is heated with hydrogen or carbon using a bunsen burner. it is not reduced to the metal, Which one is it? A. lead oxide B. Magnesium oxide C. Copper oxide D. Tin oxide Detailed SolutionThe oxides of Potassium, Sodium, Calcium, and Magnesium are not reduced when they react with carbon and hydrogen.There is an explanation video available below. |
|
| 29. |
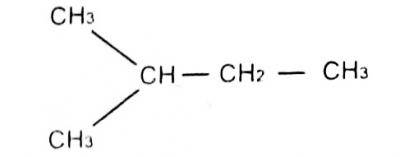 The lUPAC name for A. 1-methyl pentane B. 3-methylbutane C. 2-methylbutane D. 1-dimethyl propane |
|
| 30. |
An aqueous solution of a metal salt, M. gives a white precipitate with NaOH which dissolves in excess NaOH. With aqueous ammonia, the solution of M also gives a white precipitate which dissolves in excess ammonia Therefore the cation in M is A. Zn B. Ca C. Al D. Pb |
| 21. |
Which of the following pairs of substances will react further with oxygen to form a higher oxide? A. CO\(_2\) and H\(_2\)O B. NO and H\(_2\)O C. CO and CO\(_2\) D. SO\(_2\) and NO |
|
| 22. |
In the preparation of oxygen by heating KCIO\(_3\) , in the presence of MnO\(_2\) only moderate heat is needed because the catalyst acts by \(_2\) A. lowering the pressure of the reaction B. increasing the surface area of the reaction C. increasing the rate of the reaction D. increasing the energy barrier of the reaction |
|
| 23. |
Methanoic acid mixes with water in all proportions and has about the same boiling point as water. Which of the following methods would you adopt to obtain pure water from a mixture of Sand, water and methanoic acid? A. Extractive distillation B. Filtration followed by distillation C. Neutralization with sodium hydroxide followed by distillation D. Neutralization with sodium hydroxide followed by filtration Detailed SolutionDue to the presence of maximum Azeotrope,we use extractive distilination (with extractive distilation agents like sulfone) used for mixtures with low relative volatility,nearing unity.Azeotrope is a mixture of liquids that has a constant boiling point because the vapour has thesame composition as the mixture. There is an explanation video available below. |
|
| 24. |
A quantity of electricity liberates 3.6g of Silver from its salt. What mass of aluminium Will be liberated from its salt by the same quantity of electricity? [Al = 27, Ag = 108]. A. 2.7g B. 1.2g C. 0.9g D. 0.3g |
|
| 25. |
Suitable reagents for the laboratory preparation of nitrogen are A. sodium dioxonitrate(III) and ammonium chloride B. sodium trioxonitrate(V) and ammonium chloride C. sodium chloride and ammonium trioxonitrate(V) D. sodium chloride and ammonium di-ozonitrate(III) |
| 26. |
The number of electrons in the valence shell of an element of atomic number 14 is? A. 1 B. 2 C. 3 D. 4 |
|
| 27. |
What volume of oxygen will remain after reacting 8cm\(^3\) of hydrogen gas with 20cm\(^3\) of oxygen gas A. 10cm\(^3\) B. 12cm\(^3\) C. 14cm\(^3\) D. 16cm\(^3\) |
|
| 28. |
If one of the following oxides is heated with hydrogen or carbon using a bunsen burner. it is not reduced to the metal, Which one is it? A. lead oxide B. Magnesium oxide C. Copper oxide D. Tin oxide Detailed SolutionThe oxides of Potassium, Sodium, Calcium, and Magnesium are not reduced when they react with carbon and hydrogen.There is an explanation video available below. |
|
| 29. |
 The lUPAC name for A. 1-methyl pentane B. 3-methylbutane C. 2-methylbutane D. 1-dimethyl propane |
|
| 30. |
An aqueous solution of a metal salt, M. gives a white precipitate with NaOH which dissolves in excess NaOH. With aqueous ammonia, the solution of M also gives a white precipitate which dissolves in excess ammonia Therefore the cation in M is A. Zn B. Ca C. Al D. Pb |