Year :
2018
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
11 - 20 of 87 Questions
| # | Question | Ans |
|---|---|---|
| 11. |
Which of the following gases may not be dried with concentrated sulphuric acid? A. HCl\(_{(g)}\) B. NH\(_3\) C. Cl\(_2\) D. SO\(_2\) |
|
| 12. |
The Consecutive members of an alkane homologous series differ by A. CH B. CH\(_2\) C. CH\(_3\) D. C\(_n\)H\(_n\) |
|
| 13. |
A correct electrochemical series can be obtained from Na, Ca, Al, Mg, Zn, Fe, Pb, H, Cu, Hg, Ag, Au by interchanging A. Al and Mg B. Zn and Fe C. Zn and Pb D. Pb and H |
|
| 14. |
A basic postulate of the kinetic theory of gases is that the molecules of a gas move in straight lines between collisions. This implies that A. collisions are perfectly elastic B. forces of repulsion exist C. forces of repulsion and attraction are in equilibrium D. collisions are inelastic |
|
| 15. |
On which of the following is the solubility of a gaseous substance dependent? A. I, II, III and IV B. I and II only C. II only D. III and IV only Detailed SolutionThe solubility of a gas decreases with an increase in temperature and a decrease in pressure.There is an explanation video available below. |
|
| 16. |
Which of the following statements is correct about the periodic table? A. Elements in the same period have the same number of valence electrons B. The valence electrons of the elements in the same period increase progressively across the period C. Elements in the same group have the same number of electron shells D. The non-metallic Properties of the elements tend to decrease across each period Detailed SolutionThe valence electrons refer to the number of electrons in the outer shell. Across a period (horizontal row), the valence electron increases.There is an explanation video available below. |
|
| 17. |
The periodic classification is an arrangement of the elements A. atomic weights B. isotopic weights C. molecular weights D. atomic numbers Detailed SolutionElements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the groupElements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the group There is an explanation video available below. |
|
| 18. |
If 1 litre of 2.2M sulphuric acid is poured into a bucket containing 10 litres of water and the resulting solution mixed thoroughly, the resulting sulphuric acid concentration will be A. 2.2M B. 1.1M C. 0.2M D. 0.11M |
|
| 19. |
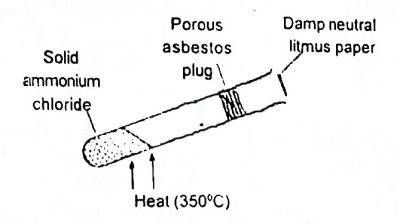 In the shown experiment (Fig. 1) the litmus paper will initially A. be bleached B. turn green C. turn red D. turn blue |
|
| 20. |
The boiling of fat and aqueous caustic soda is referred to as A. hydrolysis B. esterification C. acidification D. saponification |
| 11. |
Which of the following gases may not be dried with concentrated sulphuric acid? A. HCl\(_{(g)}\) B. NH\(_3\) C. Cl\(_2\) D. SO\(_2\) |
|
| 12. |
The Consecutive members of an alkane homologous series differ by A. CH B. CH\(_2\) C. CH\(_3\) D. C\(_n\)H\(_n\) |
|
| 13. |
A correct electrochemical series can be obtained from Na, Ca, Al, Mg, Zn, Fe, Pb, H, Cu, Hg, Ag, Au by interchanging A. Al and Mg B. Zn and Fe C. Zn and Pb D. Pb and H |
|
| 14. |
A basic postulate of the kinetic theory of gases is that the molecules of a gas move in straight lines between collisions. This implies that A. collisions are perfectly elastic B. forces of repulsion exist C. forces of repulsion and attraction are in equilibrium D. collisions are inelastic |
|
| 15. |
On which of the following is the solubility of a gaseous substance dependent? A. I, II, III and IV B. I and II only C. II only D. III and IV only Detailed SolutionThe solubility of a gas decreases with an increase in temperature and a decrease in pressure.There is an explanation video available below. |
| 16. |
Which of the following statements is correct about the periodic table? A. Elements in the same period have the same number of valence electrons B. The valence electrons of the elements in the same period increase progressively across the period C. Elements in the same group have the same number of electron shells D. The non-metallic Properties of the elements tend to decrease across each period Detailed SolutionThe valence electrons refer to the number of electrons in the outer shell. Across a period (horizontal row), the valence electron increases.There is an explanation video available below. |
|
| 17. |
The periodic classification is an arrangement of the elements A. atomic weights B. isotopic weights C. molecular weights D. atomic numbers Detailed SolutionElements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the groupElements in the periodic table are arranged according to their atomic number (proton number). Electronegativity increase from left to right while atomic radius increases down the group There is an explanation video available below. |
|
| 18. |
If 1 litre of 2.2M sulphuric acid is poured into a bucket containing 10 litres of water and the resulting solution mixed thoroughly, the resulting sulphuric acid concentration will be A. 2.2M B. 1.1M C. 0.2M D. 0.11M |
|
| 19. |
 In the shown experiment (Fig. 1) the litmus paper will initially A. be bleached B. turn green C. turn red D. turn blue |
|
| 20. |
The boiling of fat and aqueous caustic soda is referred to as A. hydrolysis B. esterification C. acidification D. saponification |