Year :
2010
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 49 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
Which of the following is used to hasten the ripening of fruit? A. Ethene B. Ethanol C. Ethyne D. Ethane Detailed SolutionEthylene is a plant hormone produced during fruit ripening which mobilizes the genes to synthesize the enzymes which are responsible for fruit ripening.There is an explanation video available below. |
|
| 42. |
The final products of the reaction between methane and chlorine in the presence of ultraviolet light are hydrogen chloride and? A. tricloromethane B. dichloromethane C. tetrachloromethane D. chloromethane Detailed SolutionCarbon tetrachloride ( IUPAC: Tetrachloromethane) CCl\(_4\), is the final product obtained in this substitution reaction.There is an explanation video available below. |
|
| 43. |
The correct order of increasing boiling points of the following compounds C3H7OH, C7H16 and C4H10 is? A. C3H7OH → C4H10 → C7H10 B. C4H10 → C7H16 → C3H7OH C. C7H16 → C3H7OH → C4H10 D. C4H10 → C3H7OH → C7H16 Detailed SolutionC3H7OH ( PROPANOL) ⇒ 97ºCC7H16 ( HEPTANE ) ⇒ 98.42ºC ;and C4H10 ( BUTANE ) ⇒ -1ºC There is an explanation video available below. |
|
| 44. |
One of the major uses of alkane is? A. as domestic and industrial fuel B. in the hydrogenation of oils C. in the textile industries D. in the production of plastics Detailed Solution- Alkanes will react with Oxygen if they are given sufficient Activation Energy. This will result in a highly Exothermic reaction, producing Carbon Dioxide and Water, which makes Alkanes very useful as fuels.There is an explanation video available below. |
|
| 45. |
The haloalkanes used in dry-cleaning industries are? A. trichloromethane and tetrachloromethane B. chloroethene and dichloroethene C. trichloroethene and tetrachloroethene D. chloroethane and dichloroethene Detailed Solution- Both trichloromethane and tetrachloromethane are such important organic solvent.There is an explanation video available below. |
|
| 46. |
Two hydrocarbons X and Y were treated with bromine water. X decolorised the solution and Y did not not. Which class of compound does Y belong? A. Benzene B. Alkynes C. Alkenes D. Alkanes Detailed SolutionBromine water is an orange solution of bromine. It becomes colorless when it is shaken with an alkene. Alkenes can decolorize bromine water, but alkanes cannot.X is ethene which decolorizes bromine water showing that ethene is unsaturated. Y is an alkane. There is an explanation video available below. |
|
| 47. |
The compound that is used as an anesthetic is? A. CCl4 B. CH Cl3 C. CH2Cl2 D. CH3Cl Detailed Solution- Chloroform / tricholoromethane ( CHCl\(_3\) ) is a potent anaesthetic agent.- Though Chloroform is no longer used as an anaesthetic for several reasons, the most important of which is the relatively high risk of complications, including possible heart failure There is an explanation video available below. |
|
| 48. |
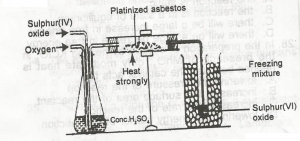 In the diagram above, the purpose of the asbestos to? A. absorb impurities B. catalyze the reaction C. solidify the gas D. dry the gas Detailed SolutionCatalyst like platinize asbestos or vanadium(v)oxide is required to speed up the combination to form sulphur(iv)oxide.There is an explanation video available below. |
|
| 49. |
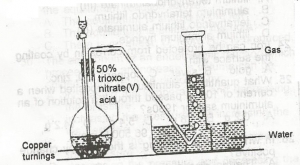 In the diagram above, the gas produced is? A. NO B. NO2 C. N2O D. N2O4 Detailed SolutionIn this laboratory preparation, 50% trioxonitrate (V) acid is reacted with copper turnings to liberate nitrogen (II) oxide ( NO ) that is collected by downward delivery.There is an explanation video available below. |
| 41. |
Which of the following is used to hasten the ripening of fruit? A. Ethene B. Ethanol C. Ethyne D. Ethane Detailed SolutionEthylene is a plant hormone produced during fruit ripening which mobilizes the genes to synthesize the enzymes which are responsible for fruit ripening.There is an explanation video available below. |
|
| 42. |
The final products of the reaction between methane and chlorine in the presence of ultraviolet light are hydrogen chloride and? A. tricloromethane B. dichloromethane C. tetrachloromethane D. chloromethane Detailed SolutionCarbon tetrachloride ( IUPAC: Tetrachloromethane) CCl\(_4\), is the final product obtained in this substitution reaction.There is an explanation video available below. |
|
| 43. |
The correct order of increasing boiling points of the following compounds C3H7OH, C7H16 and C4H10 is? A. C3H7OH → C4H10 → C7H10 B. C4H10 → C7H16 → C3H7OH C. C7H16 → C3H7OH → C4H10 D. C4H10 → C3H7OH → C7H16 Detailed SolutionC3H7OH ( PROPANOL) ⇒ 97ºCC7H16 ( HEPTANE ) ⇒ 98.42ºC ;and C4H10 ( BUTANE ) ⇒ -1ºC There is an explanation video available below. |
|
| 44. |
One of the major uses of alkane is? A. as domestic and industrial fuel B. in the hydrogenation of oils C. in the textile industries D. in the production of plastics Detailed Solution- Alkanes will react with Oxygen if they are given sufficient Activation Energy. This will result in a highly Exothermic reaction, producing Carbon Dioxide and Water, which makes Alkanes very useful as fuels.There is an explanation video available below. |
|
| 45. |
The haloalkanes used in dry-cleaning industries are? A. trichloromethane and tetrachloromethane B. chloroethene and dichloroethene C. trichloroethene and tetrachloroethene D. chloroethane and dichloroethene Detailed Solution- Both trichloromethane and tetrachloromethane are such important organic solvent.There is an explanation video available below. |
| 46. |
Two hydrocarbons X and Y were treated with bromine water. X decolorised the solution and Y did not not. Which class of compound does Y belong? A. Benzene B. Alkynes C. Alkenes D. Alkanes Detailed SolutionBromine water is an orange solution of bromine. It becomes colorless when it is shaken with an alkene. Alkenes can decolorize bromine water, but alkanes cannot.X is ethene which decolorizes bromine water showing that ethene is unsaturated. Y is an alkane. There is an explanation video available below. |
|
| 47. |
The compound that is used as an anesthetic is? A. CCl4 B. CH Cl3 C. CH2Cl2 D. CH3Cl Detailed Solution- Chloroform / tricholoromethane ( CHCl\(_3\) ) is a potent anaesthetic agent.- Though Chloroform is no longer used as an anaesthetic for several reasons, the most important of which is the relatively high risk of complications, including possible heart failure There is an explanation video available below. |
|
| 48. |
 In the diagram above, the purpose of the asbestos to? A. absorb impurities B. catalyze the reaction C. solidify the gas D. dry the gas Detailed SolutionCatalyst like platinize asbestos or vanadium(v)oxide is required to speed up the combination to form sulphur(iv)oxide.There is an explanation video available below. |
|
| 49. |
 In the diagram above, the gas produced is? A. NO B. NO2 C. N2O D. N2O4 Detailed SolutionIn this laboratory preparation, 50% trioxonitrate (V) acid is reacted with copper turnings to liberate nitrogen (II) oxide ( NO ) that is collected by downward delivery.There is an explanation video available below. |