Year :
2010
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
Which of the following is used in rocket fuels? A. HNO3 B. CH3COOH C. H2SO4 D. HCl Detailed Solution- Due to its unique powerful oxidizing nature, Nitric acid is often used in rocket fuels as a propellerNB: Ethanol (not Ethanoic acid) is used alone or mixed with petrol as fuel for racing cars and in rockets. There is an explanation video available below. |
|
| 32. |
A constituent common to bronze and solder is? A. lead B. silver C. copper D. tin Detailed Solution- bronze, alloy traditionally composed of copper and tin.- Solder is an alloy, made from the elements, tin and lead. There is an explanation video available below. |
|
| 33. |
When iron is exposed to moist air, it gradually rusts. This is due to the formation of? A. hydrate iron (III) oxide B. anhydrous iron (III) oxide C. anhydrous iron (II) oxide D. hydrate iron (II) oxide Detailed SolutionRusting is an oxidation reaction. The iron reacts with water and oxygen to form hydrated iron(III) oxide, which we see as rust.There is an explanation video available below. |
|
| 34. |
A compound gives an orange-red color to non-luminous flame. This compound is likely to contain? A. Na+ B. Ca2+ C. Fe3+ D. Fe2+ Detailed Solution- Calcium when introduced into the flame, it will produce brick red / red-orange colour.There is an explanation video available below. |
|
| 35. |
Stainless steel is used for making? A. magnets B. tools C. coins and medals D. moving parts of clocks Detailed Solution- Stainless steel is an alloy of iron, nickel and chromium. It is used in making cutlery, tools and surgical equipment.There is an explanation video available below. |
|
| 36. |
The residual solids from the fractional distillation of petroleum are used as? A. coatings of pipes B. raw materials for the cracking process C. fuel for the driving tractors D. fuel for jet engines Detailed SolutionOther residues (apart from bitumen) are used as fuel, pipe coating, etcThere is an explanation video available below. |
|
| 37. |
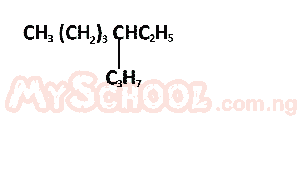 The IUPAC nomenclature of the compound above is? A. 4 - ethyloctane B. 5 - ethyloctane C. 5 - propylheptane D. 3 - propylheptane Detailed SolutionAdhering to the basic naming rules, this compound is identified as 4 - ethyloctaneThere is an explanation video available below. |
|
| 38. |
Which of the following is used as fuel in miners' lamp? A. Ethanal B. Ethyne C. Ethene D. Ethane Detailed SolutionEthyne is a sometimes used a fuel in miners'lamps.There is an explanation video available below. |
|
| 39. |
Which of the following organic compounds is very soluble in water? A. CH3COOH B. C2H2 C. C2H4 D. CH3COOC2H5 Detailed SolutionEthanoic acid ( CH\(_3\)COOH ) is the only organic compound listed here that is (very) soluble in water.There is an explanation video available below. |
|
| 40. |
Benzene reacts with hydrogen in the presence of nickel catalyst at 180ºC to give? A. xylene B. toluene C. cyclopentane D. cyclohexane Detailed SolutionCyclohexane is formed when Benzene (vapor) undergoes addition reaction with hydrogen in the presence of nickel catalyst at 180°CThere is an explanation video available below. |
| 31. |
Which of the following is used in rocket fuels? A. HNO3 B. CH3COOH C. H2SO4 D. HCl Detailed Solution- Due to its unique powerful oxidizing nature, Nitric acid is often used in rocket fuels as a propellerNB: Ethanol (not Ethanoic acid) is used alone or mixed with petrol as fuel for racing cars and in rockets. There is an explanation video available below. |
|
| 32. |
A constituent common to bronze and solder is? A. lead B. silver C. copper D. tin Detailed Solution- bronze, alloy traditionally composed of copper and tin.- Solder is an alloy, made from the elements, tin and lead. There is an explanation video available below. |
|
| 33. |
When iron is exposed to moist air, it gradually rusts. This is due to the formation of? A. hydrate iron (III) oxide B. anhydrous iron (III) oxide C. anhydrous iron (II) oxide D. hydrate iron (II) oxide Detailed SolutionRusting is an oxidation reaction. The iron reacts with water and oxygen to form hydrated iron(III) oxide, which we see as rust.There is an explanation video available below. |
|
| 34. |
A compound gives an orange-red color to non-luminous flame. This compound is likely to contain? A. Na+ B. Ca2+ C. Fe3+ D. Fe2+ Detailed Solution- Calcium when introduced into the flame, it will produce brick red / red-orange colour.There is an explanation video available below. |
|
| 35. |
Stainless steel is used for making? A. magnets B. tools C. coins and medals D. moving parts of clocks Detailed Solution- Stainless steel is an alloy of iron, nickel and chromium. It is used in making cutlery, tools and surgical equipment.There is an explanation video available below. |
| 36. |
The residual solids from the fractional distillation of petroleum are used as? A. coatings of pipes B. raw materials for the cracking process C. fuel for the driving tractors D. fuel for jet engines Detailed SolutionOther residues (apart from bitumen) are used as fuel, pipe coating, etcThere is an explanation video available below. |
|
| 37. |
 The IUPAC nomenclature of the compound above is? A. 4 - ethyloctane B. 5 - ethyloctane C. 5 - propylheptane D. 3 - propylheptane Detailed SolutionAdhering to the basic naming rules, this compound is identified as 4 - ethyloctaneThere is an explanation video available below. |
|
| 38. |
Which of the following is used as fuel in miners' lamp? A. Ethanal B. Ethyne C. Ethene D. Ethane Detailed SolutionEthyne is a sometimes used a fuel in miners'lamps.There is an explanation video available below. |
|
| 39. |
Which of the following organic compounds is very soluble in water? A. CH3COOH B. C2H2 C. C2H4 D. CH3COOC2H5 Detailed SolutionEthanoic acid ( CH\(_3\)COOH ) is the only organic compound listed here that is (very) soluble in water.There is an explanation video available below. |
|
| 40. |
Benzene reacts with hydrogen in the presence of nickel catalyst at 180ºC to give? A. xylene B. toluene C. cyclopentane D. cyclohexane Detailed SolutionCyclohexane is formed when Benzene (vapor) undergoes addition reaction with hydrogen in the presence of nickel catalyst at 180°CThere is an explanation video available below. |