Year :
2010
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
21 - 30 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 21. |
6AgNO4(aq) + PH3(g) + 3H2O(l) → 6Ag(s) + H3PO3(g) + 6HNO3(aq) A. HNO3(aq) B. H2O(l) C. PH3(g) D. AgNO3(aq) Detailed SolutionPhosphorus(iii)hydride is a strong reducing agent which reduces solution of silver or copper(ii)salts to the metals or phosphides.There is an explanation video available below. |
|
| 22. |
The IUPAC nomenclature of the compound LiAlH4 is? A. lithiumtetrahydridoaluminate (III) B. aluminium tetrahydrido lithium C. tetrahydrido lithium aluminate (III) D. lithium aluminium hydride |
|
| 23. |
Iron can be protected from corrosion by coating the surface with? A. gold B. silver C. copper D. zinc |
|
| 24. |
What quantity of aluminum is deposited when a current of 10A is passed through a solution of an aluminum salt for 1930s? A. 0.2 g B. 1.8 g C. 5.4 g D. 14.2 g |
|
| 25. |
In which of the following is the entropy change positive? A. Thermal dissociation of ammonium chloride B. Reaction between an acid and a base C. Addition of concentrated acid to water D. Dissolution of sodium metal in water Detailed SolutionThermal dissociation of ammonium chloride is an endothermic reaction as heat is absorbed for a positive entropy, with more products than reactants.There is an explanation video available below. |
|
| 26. |
If a reaction is exothermic and there is a great disorder, it means that? A. the reaction is static B. the reaction is in a state of equilibrium C. there will be a large increase in free energy D. there will be a large decrease in free energy Detailed SolutionIf a reaction is exothermic and there is a great disorder for a spontaneous reaction to occur, a large decrease in free energy is evident.There is an explanation video available below. |
|
| 27. |
In the preparation of oxygen by heating KClO3 in the presence of MnO2, only moderate heat is needed because the catalyst acts by? A. lowering the pressure of the reaction B. increasing the surface area of the reactant C. increase the rate of the reaction D. lowering the energy barrier of the reaction Detailed Solution- The presence of MnO2 increase the rate of the reaction by providing a new reaction path / making more particles possess kinetic energy greater then the activation energy.There is an explanation video available below. |
|
| 28. |
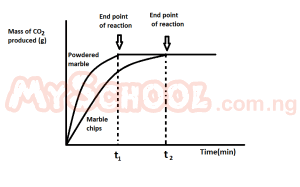 The graph above demonstrate the effect of? A. surface area on the rate of reaction B. catalyst on the rate of reaction C. pressure on the rate reaction D. concentration on the rate of reaction |
|
| 29. |
2H2(g) + O2(g) ⇌ 2H2O(g) ΔH = -ve A. it is unaffected B. it becomes zero C. it decrease D. it increase Detailed SolutionThis given equation shows the forward reaction is exothermic, which means an increase in temperature will cause the equilibrium position to shift to left to favor reactant formation, i.e K decreasesThere is an explanation video available below. |
|
| 30. |
To a solution of an unknown compound, a little dilute tetraoxosulphate (VI) acid was added with some freshly prepared iron (II) tetraoxosulphate (VI) solution. The brown ring observed after the addition of a stream of concentrated tetraoxosulphate (VI) acid confirmed the presence of? A. CO\(^{2-}_{3}\) B. Cl- C. SO\(_{3}^{2-}\) D. NO\(^{2-}_{3}\) Detailed SolutionNO\(_{3}^{-}\) is confirmed, then the brown ring is due to the formation of FESO\(_4\).NOThere is an explanation video available below. |
| 21. |
6AgNO4(aq) + PH3(g) + 3H2O(l) → 6Ag(s) + H3PO3(g) + 6HNO3(aq) A. HNO3(aq) B. H2O(l) C. PH3(g) D. AgNO3(aq) Detailed SolutionPhosphorus(iii)hydride is a strong reducing agent which reduces solution of silver or copper(ii)salts to the metals or phosphides.There is an explanation video available below. |
|
| 22. |
The IUPAC nomenclature of the compound LiAlH4 is? A. lithiumtetrahydridoaluminate (III) B. aluminium tetrahydrido lithium C. tetrahydrido lithium aluminate (III) D. lithium aluminium hydride |
|
| 23. |
Iron can be protected from corrosion by coating the surface with? A. gold B. silver C. copper D. zinc |
|
| 24. |
What quantity of aluminum is deposited when a current of 10A is passed through a solution of an aluminum salt for 1930s? A. 0.2 g B. 1.8 g C. 5.4 g D. 14.2 g |
|
| 25. |
In which of the following is the entropy change positive? A. Thermal dissociation of ammonium chloride B. Reaction between an acid and a base C. Addition of concentrated acid to water D. Dissolution of sodium metal in water Detailed SolutionThermal dissociation of ammonium chloride is an endothermic reaction as heat is absorbed for a positive entropy, with more products than reactants.There is an explanation video available below. |
| 26. |
If a reaction is exothermic and there is a great disorder, it means that? A. the reaction is static B. the reaction is in a state of equilibrium C. there will be a large increase in free energy D. there will be a large decrease in free energy Detailed SolutionIf a reaction is exothermic and there is a great disorder for a spontaneous reaction to occur, a large decrease in free energy is evident.There is an explanation video available below. |
|
| 27. |
In the preparation of oxygen by heating KClO3 in the presence of MnO2, only moderate heat is needed because the catalyst acts by? A. lowering the pressure of the reaction B. increasing the surface area of the reactant C. increase the rate of the reaction D. lowering the energy barrier of the reaction Detailed Solution- The presence of MnO2 increase the rate of the reaction by providing a new reaction path / making more particles possess kinetic energy greater then the activation energy.There is an explanation video available below. |
|
| 28. |
 The graph above demonstrate the effect of? A. surface area on the rate of reaction B. catalyst on the rate of reaction C. pressure on the rate reaction D. concentration on the rate of reaction |
|
| 29. |
2H2(g) + O2(g) ⇌ 2H2O(g) ΔH = -ve A. it is unaffected B. it becomes zero C. it decrease D. it increase Detailed SolutionThis given equation shows the forward reaction is exothermic, which means an increase in temperature will cause the equilibrium position to shift to left to favor reactant formation, i.e K decreasesThere is an explanation video available below. |
|
| 30. |
To a solution of an unknown compound, a little dilute tetraoxosulphate (VI) acid was added with some freshly prepared iron (II) tetraoxosulphate (VI) solution. The brown ring observed after the addition of a stream of concentrated tetraoxosulphate (VI) acid confirmed the presence of? A. CO\(^{2-}_{3}\) B. Cl- C. SO\(_{3}^{2-}\) D. NO\(^{2-}_{3}\) Detailed SolutionNO\(_{3}^{-}\) is confirmed, then the brown ring is due to the formation of FESO\(_4\).NOThere is an explanation video available below. |