Year :
2008
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
Which of the following compounds of trioxonitrate (V) will decomposes to give dinitrogen (i) oxide and water when heated? A. NaNO3 B. Zn(NO3)2 C. Cu(NO3)2 D. NH4NO3 |
D |
| 32. |
When a solution of ammonium trioxocarbonate (IV) is added to a solution of an unknown salt, a white precipitate which is soluble in dilute hydrochloric acid but insoluble in ethanoic acid is formed. This indicates the presence of A. Ca2+ B. Na+ C. Zn2+ D. K+ |
A |
| 33. |
Leads is used for making bullets and lead shots because of its A. resistance to corrosion B. low melting point C. high density D. flexibility |
C |
| 34. |
Which of the following gives a precipitate when treated with NaOH solution? A. AlCl3 B. NH4Cl C. Na2CO3 D. CH3COONa |
A |
| 35. |
The most common ores of iron include A. haematite, malachite and limonite B. chalcolite, calamine and bornite C. magnetite, haematite and bornite D. magnetite, chalcolite and bornite |
C |
| 36. |
The type of isomerism shown by cis- and trans- isomers is A. optical isomerism B. positional isomerism C. functional isomerism D. geometrical isomerism |
D |
| 37. |
If the silver mirror test is positive, it indicates the presence of an A. alkyne B. alkanol C. alkanone D. alkanal |
D |
| 38. |
If glucose is heated with concentration tetraoxosulphate (VI) acid, it will be dehydrated to A. carbon B. carbon (IV) oxide C. ethene D. ethanol |
A |
| 39. |
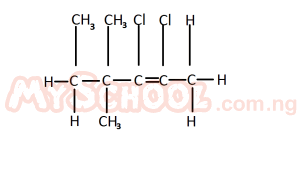 The IUPAC nomenclature for the structure above is A. 2, 3 - dichloro - 4, 4, 5 - trimethyl pent - 2 - ene B. 4, 5 - dichloro - 2, 3 - dimethyl hex - 2 - ene C. 2, 3 - dichloro - 4, 4 - dimethyl hex - 2- ene D. 2, 3 dichloro - 2, 2 - dimethyl hex - 2 - ene Detailed SolutionThe longest carbon chain in the compound is the pent-2-ene.Counting all the attachments, the IUPAC nomenclature of the compound is 2,3 - dichloro-4,4,5 - trimethyl pent-2-ene. |
|
| 40. |
A hydrocarbon X with a molar mass of 26 consists of 92.3% carbon. What is its molecular formular? A. C2H2 B. C3H3 C. C4H4 D. C5H5 Detailed Solution% of H2 = 100 - 92.3 = 7.7%mole = Carbon [92.3 / 12] = 7.7 Hydrogen [7.7 / 1] = 7.7 divide with the lowest: Carbon - 7.1 / 7.1 = 1 Hydrogen - 7.1 / 7.1 = 1 empirical formula = CH (CH)n = 26 (12 + 1)n = 26 (13)n = 26 n = 26/13 = 2 molecular formula = C\(_2\)H\(_2\) |
| 31. |
Which of the following compounds of trioxonitrate (V) will decomposes to give dinitrogen (i) oxide and water when heated? A. NaNO3 B. Zn(NO3)2 C. Cu(NO3)2 D. NH4NO3 |
D |
| 32. |
When a solution of ammonium trioxocarbonate (IV) is added to a solution of an unknown salt, a white precipitate which is soluble in dilute hydrochloric acid but insoluble in ethanoic acid is formed. This indicates the presence of A. Ca2+ B. Na+ C. Zn2+ D. K+ |
A |
| 33. |
Leads is used for making bullets and lead shots because of its A. resistance to corrosion B. low melting point C. high density D. flexibility |
C |
| 34. |
Which of the following gives a precipitate when treated with NaOH solution? A. AlCl3 B. NH4Cl C. Na2CO3 D. CH3COONa |
A |
| 35. |
The most common ores of iron include A. haematite, malachite and limonite B. chalcolite, calamine and bornite C. magnetite, haematite and bornite D. magnetite, chalcolite and bornite |
C |
| 36. |
The type of isomerism shown by cis- and trans- isomers is A. optical isomerism B. positional isomerism C. functional isomerism D. geometrical isomerism |
D |
| 37. |
If the silver mirror test is positive, it indicates the presence of an A. alkyne B. alkanol C. alkanone D. alkanal |
D |
| 38. |
If glucose is heated with concentration tetraoxosulphate (VI) acid, it will be dehydrated to A. carbon B. carbon (IV) oxide C. ethene D. ethanol |
A |
| 39. |
 The IUPAC nomenclature for the structure above is A. 2, 3 - dichloro - 4, 4, 5 - trimethyl pent - 2 - ene B. 4, 5 - dichloro - 2, 3 - dimethyl hex - 2 - ene C. 2, 3 - dichloro - 4, 4 - dimethyl hex - 2- ene D. 2, 3 dichloro - 2, 2 - dimethyl hex - 2 - ene Detailed SolutionThe longest carbon chain in the compound is the pent-2-ene.Counting all the attachments, the IUPAC nomenclature of the compound is 2,3 - dichloro-4,4,5 - trimethyl pent-2-ene. |
|
| 40. |
A hydrocarbon X with a molar mass of 26 consists of 92.3% carbon. What is its molecular formular? A. C2H2 B. C3H3 C. C4H4 D. C5H5 Detailed Solution% of H2 = 100 - 92.3 = 7.7%mole = Carbon [92.3 / 12] = 7.7 Hydrogen [7.7 / 1] = 7.7 divide with the lowest: Carbon - 7.1 / 7.1 = 1 Hydrogen - 7.1 / 7.1 = 1 empirical formula = CH (CH)n = 26 (12 + 1)n = 26 (13)n = 26 n = 26/13 = 2 molecular formula = C\(_2\)H\(_2\) |