Year :
2011
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
1 - 10 of 47 Questions
| # | Question | Ans |
|---|---|---|
| 1. |
What is the concentration of a solution containing 2g of NaOH in 100cm3 of solution? A. 0.40 mol dm-3 B. 0.50 mol dm-3 C. 0.05 mol dm-3 D. 0.30 mol dm-3 |
|
| 2. |
Which of the following properties is NOT peculiar to matter? A. kinetic energy of particles increase from solid to gas B. Random motion of particles increases from liquid to gas C. Orderliness of particles increases from gas to liquid D. Random motion of particles increases from gas to solid |
|
| 3. |
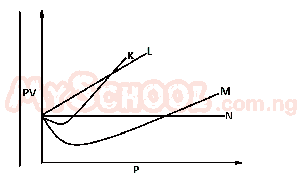 From the diagram above, an ideal can be represented by A. M B. N C. K D. L |
|
| 4. |
Which of the following questions is correct about the periodic table? A. The non-metallic properties of the elements tend to decrease across each period B. The valence electrons of the elements increase progressively across the period C. Elements in the same group have the same numbeer of electron shells D. Elements in the same period have the number of valence electrons |
|
| 5. |
The relative atomic mass of a naturally occurring lithium consisting of 90% \(^7 _3\)Li and \(^6 _3\)10% Li is A. 6.9 B. 7.1 C. 6.2 D. 6.8 Detailed SolutionR.A.M = m\(_1 \alpha_1\) + m\(_2 \alpha_2\)= 7(90/100) + 6(10/100) = 6.3 + 0.6 = 6.9 There is an explanation video available below. |
|
| 6. |
An isotope has an atomic number of 15 and a mass number of 31. The number of protons it contain is A. 16 B. 15 C. 46 D. 31 |
|
| 7. |
The molecular lattice of iodine is held together by A. dative bond B. metallic bond C. hydrogen bond D. van der Waal's forces |
|
| 8. |
The arrangement of particles in crystal lattices can be studied using A. X - rays B. γ - rays C. α - rays D. β - rays |
|
| 9. |
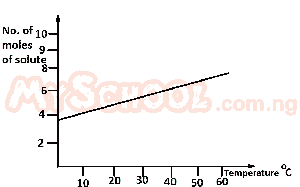 From the diagram above, find the amount of solute deposited when 200 cm\(^3\) of the solution is cooled from 55°C to 40°C. A. 0.10 mole B. 0.20mole C. 0.01 mole D. 0.02 mole Detailed SolutionFrom the diagram,55°C = 7 moles; 40°C = 6 moles. Amount of solute deposited = 7 - 6 = 1 mole. 1000 cm\(^3\) = 1 mole 200 cm\(^3\) = x x = \(\frac{200 \times 1}{1000}\) = 0.20 mole. There is an explanation video available below. |
|
| 10. |
The importance of sodium aluminate (III) in the treatment of water is to A. cause coagulation B. neutralize acidity C. prevent goitre and tooth decay D. kill germs |
| 1. |
What is the concentration of a solution containing 2g of NaOH in 100cm3 of solution? A. 0.40 mol dm-3 B. 0.50 mol dm-3 C. 0.05 mol dm-3 D. 0.30 mol dm-3 |
|
| 2. |
Which of the following properties is NOT peculiar to matter? A. kinetic energy of particles increase from solid to gas B. Random motion of particles increases from liquid to gas C. Orderliness of particles increases from gas to liquid D. Random motion of particles increases from gas to solid |
|
| 3. |
 From the diagram above, an ideal can be represented by A. M B. N C. K D. L |
|
| 4. |
Which of the following questions is correct about the periodic table? A. The non-metallic properties of the elements tend to decrease across each period B. The valence electrons of the elements increase progressively across the period C. Elements in the same group have the same numbeer of electron shells D. Elements in the same period have the number of valence electrons |
|
| 5. |
The relative atomic mass of a naturally occurring lithium consisting of 90% \(^7 _3\)Li and \(^6 _3\)10% Li is A. 6.9 B. 7.1 C. 6.2 D. 6.8 Detailed SolutionR.A.M = m\(_1 \alpha_1\) + m\(_2 \alpha_2\)= 7(90/100) + 6(10/100) = 6.3 + 0.6 = 6.9 There is an explanation video available below. |
| 6. |
An isotope has an atomic number of 15 and a mass number of 31. The number of protons it contain is A. 16 B. 15 C. 46 D. 31 |
|
| 7. |
The molecular lattice of iodine is held together by A. dative bond B. metallic bond C. hydrogen bond D. van der Waal's forces |
|
| 8. |
The arrangement of particles in crystal lattices can be studied using A. X - rays B. γ - rays C. α - rays D. β - rays |
|
| 9. |
 From the diagram above, find the amount of solute deposited when 200 cm\(^3\) of the solution is cooled from 55°C to 40°C. A. 0.10 mole B. 0.20mole C. 0.01 mole D. 0.02 mole Detailed SolutionFrom the diagram,55°C = 7 moles; 40°C = 6 moles. Amount of solute deposited = 7 - 6 = 1 mole. 1000 cm\(^3\) = 1 mole 200 cm\(^3\) = x x = \(\frac{200 \times 1}{1000}\) = 0.20 mole. There is an explanation video available below. |
|
| 10. |
The importance of sodium aluminate (III) in the treatment of water is to A. cause coagulation B. neutralize acidity C. prevent goitre and tooth decay D. kill germs |