Year :
1993
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 49 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
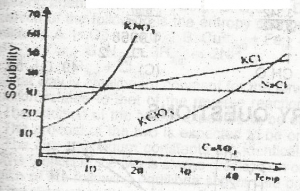 For which salt in the graph above does the solubility increase most rapidly with rise in temperature? A. CaSO4 B. KNO3 C. NaCl D. KCl |
B |
| 42. |
 Half-cell reaction. From the data above , it can be deduced that the most powerful reducing agent of the four metals is A. Cu B. Fe C. Ba D. Zn |
C |
| 43. |
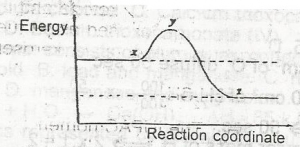 In the diagram above, curve X represents the energy profile for a homogeneous gaseous reaction. Which of the following conditions would produce curve Y for the same reaction? A. Increase in temperature B. Increase in the concentration of a reactant C. Addition of a catalyst D. Increase in pressure |
C |
| 44. |
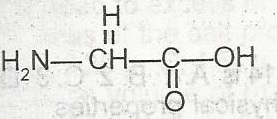 The two functional groups in the above compound are A. alcohol and amine B. acid and amine C. aldehyde and acid D. ketone and amine |
B |
| 45. |
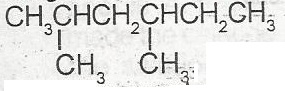 The IUPAC nomenclature for the compound above is A. dimethlhexane B. 3,5 dimethylhexane C. 1.1 dmethyl, 3 methylpentane D. 2.4 dimethylhexane |
D |
| 46. |
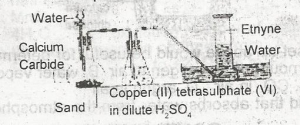 The function of the copper (ll)tetraoxosulphate (IV) in dilute H2SO4 in the figure above is to A. dry the gas B. absorb phosphine impurity C. absorb ethene impurity D. from an acetylide with ethyne |
B |
| 47. |
The dissolution of common salt in water is a physical change because A. the salt can be obtained by crystallization B. the salt can be recovered by the evaporation of water C. heat is not generated during mixing D. the solution will not boil at 100oC Detailed SolutionThe evaporation of water is a physical change. When water evaporates, it changes from the liquid state to the gas state, but it is still water; it has not changed into any other substance. |
|
| 48. |
At 25oC and 1atm, a gas occupies a volume of 1.50dm3. What volume will it occupy at 100oC and 1atm? A. 1.88 dm3 B. 6.00 dm3 C. 18.80 dm3 D. 18.80 dm3 E. 60.00dm3 Detailed Solution\(\frac{v_1}{T_1} = \frac{V_2}{T_2}\)V2 = \(\frac{V_1 \times T_2}{T_1}\) = \(\frac{1.50 \times 373}{298}\) = 1.88 dm3 |
|
| 49. |
The fraction of crude oil used as jet fuel is A. refinery gas B. diesel oil C. kerosine D. gasoline |
C |
| 41. |
 For which salt in the graph above does the solubility increase most rapidly with rise in temperature? A. CaSO4 B. KNO3 C. NaCl D. KCl |
B |
| 42. |
 Half-cell reaction. From the data above , it can be deduced that the most powerful reducing agent of the four metals is A. Cu B. Fe C. Ba D. Zn |
C |
| 43. |
 In the diagram above, curve X represents the energy profile for a homogeneous gaseous reaction. Which of the following conditions would produce curve Y for the same reaction? A. Increase in temperature B. Increase in the concentration of a reactant C. Addition of a catalyst D. Increase in pressure |
C |
| 44. |
 The two functional groups in the above compound are A. alcohol and amine B. acid and amine C. aldehyde and acid D. ketone and amine |
B |
| 45. |
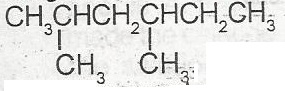 The IUPAC nomenclature for the compound above is A. dimethlhexane B. 3,5 dimethylhexane C. 1.1 dmethyl, 3 methylpentane D. 2.4 dimethylhexane |
D |
| 46. |
 The function of the copper (ll)tetraoxosulphate (IV) in dilute H2SO4 in the figure above is to A. dry the gas B. absorb phosphine impurity C. absorb ethene impurity D. from an acetylide with ethyne |
B |
| 47. |
The dissolution of common salt in water is a physical change because A. the salt can be obtained by crystallization B. the salt can be recovered by the evaporation of water C. heat is not generated during mixing D. the solution will not boil at 100oC Detailed SolutionThe evaporation of water is a physical change. When water evaporates, it changes from the liquid state to the gas state, but it is still water; it has not changed into any other substance. |
|
| 48. |
At 25oC and 1atm, a gas occupies a volume of 1.50dm3. What volume will it occupy at 100oC and 1atm? A. 1.88 dm3 B. 6.00 dm3 C. 18.80 dm3 D. 18.80 dm3 E. 60.00dm3 Detailed Solution\(\frac{v_1}{T_1} = \frac{V_2}{T_2}\)V2 = \(\frac{V_1 \times T_2}{T_1}\) = \(\frac{1.50 \times 373}{298}\) = 1.88 dm3 |
|
| 49. |
The fraction of crude oil used as jet fuel is A. refinery gas B. diesel oil C. kerosine D. gasoline |
C |