Year :
1993
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
The fraction of crude oil used as jet fuel is? A. refinery gas B. diesel oil C. kerosene D. gasoline |
C |
| 32. |
It is not desirable to use lead tetraethyl as an anti-knock agent because? A. it is expensive B. of pollution effects from the exhaust fumes C. it lowers the octane rating of petrol D. it is explosive |
B |
| 33. |
Al2O3(s) + 3H2SO4(aq) → Al2(SO4(aq)) + 3H2(I)O A. an acidic oxide B. an amphoteric oxide C. a basic oxide D. a neutral oxide |
B |
| 34. |
The carbon atoms on ethane are? A. sp2 hybridized B. sp3 hybridized C. sp2d hybridized D. sp hybridized |
B |
| 35. |
Palm wine turns sour with time because? A. the sugar content is converted into alcohol B. the carbon (IV) oxide formed during the fermentation process has a sour taste C. it is commonly adulterated by the tappers and sellers D. microbial activity results in the production of orgain acids within it |
D |
| 36. |
Catalytic hydrogenation of benzene produces? A. an aromatic hydrocarbon B. margarine C. cyclohexane D. D.D.T |
C |
| 37. |
Which of the following represents saponification? A. Reaction of carboxylic acids with sodium hydroxide B. Reaction of alkanoates with acid C. Reaction of alkanoates with alcohols D. Reaction of alkanoates with sodium hydroxide |
D |
| 38. |
The confirmatory test for alkanoic acids in organic qualitative analysis is the? A. turning of wet blue litmus paper red B. reaction with alkanols to form esters C. reaction with sodium hydroxide to form salt and water D. reaction with aqueous Na2COsp3 to liberate a gas which turns lime water milky |
B |
| 39. |
A gas that will turn orange potassium heptaoxodichromate (lV) solution to clear green is? A. sulphur (ii) oxide B. hydrogen sulphide C. sulphide (lV) oxide D. hydrogen chloride |
A |
| 40. |
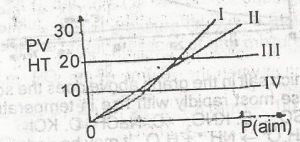 Which of the curves above represents the behaviour of 1 mole of an ideal gas? A. l B. ll C. lll D. lV |
C |
| 31. |
The fraction of crude oil used as jet fuel is? A. refinery gas B. diesel oil C. kerosene D. gasoline |
C |
| 32. |
It is not desirable to use lead tetraethyl as an anti-knock agent because? A. it is expensive B. of pollution effects from the exhaust fumes C. it lowers the octane rating of petrol D. it is explosive |
B |
| 33. |
Al2O3(s) + 3H2SO4(aq) → Al2(SO4(aq)) + 3H2(I)O A. an acidic oxide B. an amphoteric oxide C. a basic oxide D. a neutral oxide |
B |
| 34. |
The carbon atoms on ethane are? A. sp2 hybridized B. sp3 hybridized C. sp2d hybridized D. sp hybridized |
B |
| 35. |
Palm wine turns sour with time because? A. the sugar content is converted into alcohol B. the carbon (IV) oxide formed during the fermentation process has a sour taste C. it is commonly adulterated by the tappers and sellers D. microbial activity results in the production of orgain acids within it |
D |
| 36. |
Catalytic hydrogenation of benzene produces? A. an aromatic hydrocarbon B. margarine C. cyclohexane D. D.D.T |
C |
| 37. |
Which of the following represents saponification? A. Reaction of carboxylic acids with sodium hydroxide B. Reaction of alkanoates with acid C. Reaction of alkanoates with alcohols D. Reaction of alkanoates with sodium hydroxide |
D |
| 38. |
The confirmatory test for alkanoic acids in organic qualitative analysis is the? A. turning of wet blue litmus paper red B. reaction with alkanols to form esters C. reaction with sodium hydroxide to form salt and water D. reaction with aqueous Na2COsp3 to liberate a gas which turns lime water milky |
B |
| 39. |
A gas that will turn orange potassium heptaoxodichromate (lV) solution to clear green is? A. sulphur (ii) oxide B. hydrogen sulphide C. sulphide (lV) oxide D. hydrogen chloride |
A |
| 40. |
 Which of the curves above represents the behaviour of 1 mole of an ideal gas? A. l B. ll C. lll D. lV |
C |