Year :
2017
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
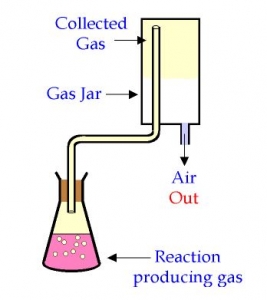
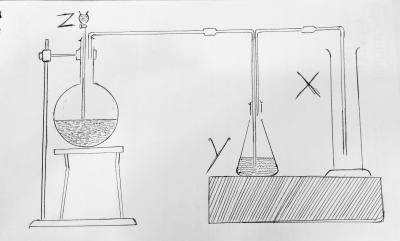 The diagram above. Y is A. fused CaO B. H2O C. NaOH D. Concentrated H2SO4 Detailed SolutionIn the preparation of sulphur dioxide by the action of dilute acids on sulphates and bisulphites. conc H2SO4 helps to release SO2 from the mixture.The setup represents the production of sulphur dioxide There is an explanation video available below. |
|
| 32. |
Calculate the mass of copper deposited when a current of 0.5 ampere was passed through a solution of copper(II) chloride for 45 minutes in an electrolytic cell. [Cu = 64, F = 96500Cmol-1] A. 0.300g B. 0.250g C. 0.2242g D. 0.448g |
|
| 33. |
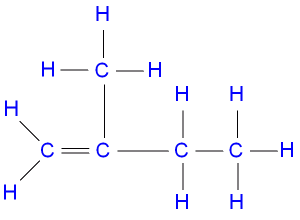 The IUPAC nomenclature of the structure above is A. 3-methybut-3-ene B. 2-methylbut-1-ene C. 2-ethylprop-1-ene D. 2-methylbut-2-ene Detailed SolutionCH2CH2 - CCH3CH2Start the numbering from the terminal carbon. There is an explanation video available below. |
|
| 34. |
The reddish–brown rust on iron roofing sheets consists of A. Fe3 + (H2O)6 B. FeO.H2O C. Fe2O3.XH2O D. Fe3O4.2H22O Detailed SolutionIron [Fe] reacts with H2 in the presence of oxygen to form a rust.4Fe + 3O2 → 2 Fe2O2 Fe2O3 + H2O → Fe2O3.H2O There is an explanation video available below. |
|
| 35. |
The densities of two gases, X and Y are 0.5gdm-3 and 2.0gdm-3 respectively. What is the rate of diffusion of X relative to Y? A. 0.1 B. 0.5 C. 2.0 D. 4.0 |
|
| 36. |
The carbon atoms on ethane are A. sp2 hybridized B. sp3 hybridized C. sp4 hybridized D. sp hybridized |
|
| 37. |
According to Charle's law, the volume of a gas becomes zero at A. −100°c B. −273°c C. −373°c D. 0°c |
|
| 38. |
An oxide XO2 has a vapour density of 32. What is the atomic mass of X? A. 20 B. 32 C. 14 D. 12 |
|
| 39. |
The gas that can be collected by downward displacement of air is A. chlorine B. sulphur (IV) oxide C. carbon (IV) oxide D. ammonia |
|
| 40. |
In the laboratory preparation of trioxonitrate (V) acid the nitrogen(iv) oxide formed as a by-product is removed by A. further heating B. adding concentrated H2SO4 C. cooling the acid solution with cold water D. bubbling air through the acid solution |
| 31. |
 The diagram above. Y is A. fused CaO B. H2O C. NaOH D. Concentrated H2SO4 Detailed SolutionIn the preparation of sulphur dioxide by the action of dilute acids on sulphates and bisulphites. conc H2SO4 helps to release SO2 from the mixture.The setup represents the production of sulphur dioxide There is an explanation video available below. |
|
| 32. |
Calculate the mass of copper deposited when a current of 0.5 ampere was passed through a solution of copper(II) chloride for 45 minutes in an electrolytic cell. [Cu = 64, F = 96500Cmol-1] A. 0.300g B. 0.250g C. 0.2242g D. 0.448g |
|
| 33. |
 The IUPAC nomenclature of the structure above is A. 3-methybut-3-ene B. 2-methylbut-1-ene C. 2-ethylprop-1-ene D. 2-methylbut-2-ene Detailed SolutionCH2CH2 - CCH3CH2Start the numbering from the terminal carbon. There is an explanation video available below. |
|
| 34. |
The reddish–brown rust on iron roofing sheets consists of A. Fe3 + (H2O)6 B. FeO.H2O C. Fe2O3.XH2O D. Fe3O4.2H22O Detailed SolutionIron [Fe] reacts with H2 in the presence of oxygen to form a rust.4Fe + 3O2 → 2 Fe2O2 Fe2O3 + H2O → Fe2O3.H2O There is an explanation video available below. |
|
| 35. |
The densities of two gases, X and Y are 0.5gdm-3 and 2.0gdm-3 respectively. What is the rate of diffusion of X relative to Y? A. 0.1 B. 0.5 C. 2.0 D. 4.0 |
| 36. |
The carbon atoms on ethane are A. sp2 hybridized B. sp3 hybridized C. sp4 hybridized D. sp hybridized |
|
| 37. |
According to Charle's law, the volume of a gas becomes zero at A. −100°c B. −273°c C. −373°c D. 0°c |
|
| 38. |
An oxide XO2 has a vapour density of 32. What is the atomic mass of X? A. 20 B. 32 C. 14 D. 12 |
|
| 39. |
The gas that can be collected by downward displacement of air is A. chlorine B. sulphur (IV) oxide C. carbon (IV) oxide D. ammonia |
|
| 40. |
In the laboratory preparation of trioxonitrate (V) acid the nitrogen(iv) oxide formed as a by-product is removed by A. further heating B. adding concentrated H2SO4 C. cooling the acid solution with cold water D. bubbling air through the acid solution |