Year :
2015
Title :
Chemistry
Exam :
WASSCE/WAEC MAY/JUNE
Paper 1 | Objectives
21 - 30 of 50 Questions
| # | Question | Ans |
|---|---|---|
| 21. |
The number of oxygen molecules present in 16.0g of the gas is ______? A. 6.02 x 1022 B. 6.02 x 1023 C. 3.01 x 1023 D. 1.51 x 1023 Detailed Solution1 mole = Molar mass of gas = Avogadros number32g of O2 = 6.02 x 1023 16g of O2 = x x = \(\frac{16 \times 6.02 \times 10^23}{32}\) = 3.01 x 1023 |
|
| 22. |
Consider the following reaction equation: A. 2 B. 3 C. 4 D. 6 Detailed Solutionfrom the reaction, the total charge on the left side = The total charge on the right side-2 + 2 + y = -2 : y = -2 : y = 2 |
|
| 23. |
The general gas equation was deprived from______? A. Boyle's and Gay Lussac's laws B. Boyle's and Graham's laws C. Boyle's and Charles' laws D. Dalton's atomic theory |
C |
| 24. |
The vapour pressure of a liquid depends on: A. I B. I and II C. I and III D. II and III |
C |
| 25. |
Which of the following gases will diffuse most rapidly? A. Cl2 B. SO2 C. CH4 D. C2H6 |
C |
| 26. |
When a reaction is endothermic, A. Enthalpy change, \(\Delta\)H is negative B. Heat content of a product is less than the heat content of a reactant C. Heat content of reactants is less than the heat content of product D. The reaction is non-spontaneous Detailed SolutionAn endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur. |
|
| 27. |
Which of the following statements about intermolecular distances and cohesive forces between gas is correct? They are A. Both large B. Both negligible C. Constant and negligible D. Large and negligible |
B |
| 28. |
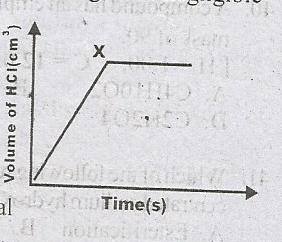 The following diagram illustrates the rate curve that was obtained when Mg reacted with excess dilute HCl. A. The reaction was slowed down B. All the dilute HCl has reacted C. All the Mg has reacted D. Hydrogen gas is produced at a steady rate |
D |
| 29. |
An example of an acid salt is A. CH3COOONa B. Mg(OH)Cl C. NaHSO4 D. (NH4)2SO4 Detailed SolutionAcidic Salt: A normal salt which is formed by the neutralization of a strong acid and weak base is called acidic salt |
|
| 30. |
Which of the following oxides can be reduced by hydrogen? A. Aluminium oxide B. Magnesium oxide C. Sodium oxide D. Silver oxide |
D |
| 21. |
The number of oxygen molecules present in 16.0g of the gas is ______? A. 6.02 x 1022 B. 6.02 x 1023 C. 3.01 x 1023 D. 1.51 x 1023 Detailed Solution1 mole = Molar mass of gas = Avogadros number32g of O2 = 6.02 x 1023 16g of O2 = x x = \(\frac{16 \times 6.02 \times 10^23}{32}\) = 3.01 x 1023 |
|
| 22. |
Consider the following reaction equation: A. 2 B. 3 C. 4 D. 6 Detailed Solutionfrom the reaction, the total charge on the left side = The total charge on the right side-2 + 2 + y = -2 : y = -2 : y = 2 |
|
| 23. |
The general gas equation was deprived from______? A. Boyle's and Gay Lussac's laws B. Boyle's and Graham's laws C. Boyle's and Charles' laws D. Dalton's atomic theory |
C |
| 24. |
The vapour pressure of a liquid depends on: A. I B. I and II C. I and III D. II and III |
C |
| 25. |
Which of the following gases will diffuse most rapidly? A. Cl2 B. SO2 C. CH4 D. C2H6 |
C |
| 26. |
When a reaction is endothermic, A. Enthalpy change, \(\Delta\)H is negative B. Heat content of a product is less than the heat content of a reactant C. Heat content of reactants is less than the heat content of product D. The reaction is non-spontaneous Detailed SolutionAn endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur. |
|
| 27. |
Which of the following statements about intermolecular distances and cohesive forces between gas is correct? They are A. Both large B. Both negligible C. Constant and negligible D. Large and negligible |
B |
| 28. |
 The following diagram illustrates the rate curve that was obtained when Mg reacted with excess dilute HCl. A. The reaction was slowed down B. All the dilute HCl has reacted C. All the Mg has reacted D. Hydrogen gas is produced at a steady rate |
D |
| 29. |
An example of an acid salt is A. CH3COOONa B. Mg(OH)Cl C. NaHSO4 D. (NH4)2SO4 Detailed SolutionAcidic Salt: A normal salt which is formed by the neutralization of a strong acid and weak base is called acidic salt |
|
| 30. |
Which of the following oxides can be reduced by hydrogen? A. Aluminium oxide B. Magnesium oxide C. Sodium oxide D. Silver oxide |
D |