Year :
1988
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 45 of 45 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
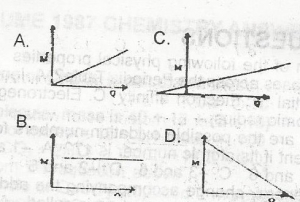 The mass of a substance, M, liberated at an electrode during electrolysis is proportional to the quantity of electricity, Q passing through the electrolyte. This is represented graphically by. A. A B. B C. C D. D |
A |
| 42. |
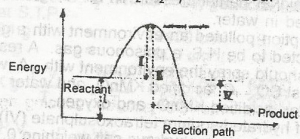 The diagram above shows the reaction path of an exothermic reaction. The heat of reaction is represented by A. l B. ll C. lll D. lV |
D |
| 43. |
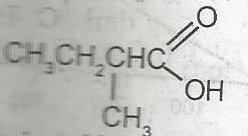 The IUPAC name for A. 2-methylbutanoic acid B. 2-methyl-1- hydroxyketone C. 2-methyl-1-hydroxy aldehyde D. 2-methylpentanoic acid |
A |
| 44. |
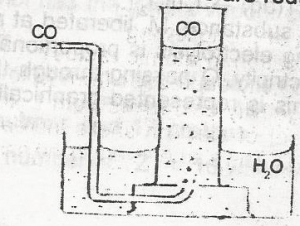 Carbon (ll) oxide may be collected as shown above because it A. is heavier than air B. is less dense than air C. is insoluble in water D. burns in oxygen to form carbon (IV) oxide |
C |
| 45. |
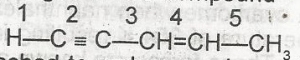 The acidic hydrogen in the compound is the hydrogen attached to carbon number A. 5 B. 4 C. 3 D. 1 |
D |
| 41. |
 The mass of a substance, M, liberated at an electrode during electrolysis is proportional to the quantity of electricity, Q passing through the electrolyte. This is represented graphically by. A. A B. B C. C D. D |
A |
| 42. |
 The diagram above shows the reaction path of an exothermic reaction. The heat of reaction is represented by A. l B. ll C. lll D. lV |
D |
| 43. |
 The IUPAC name for A. 2-methylbutanoic acid B. 2-methyl-1- hydroxyketone C. 2-methyl-1-hydroxy aldehyde D. 2-methylpentanoic acid |
A |
| 44. |
 Carbon (ll) oxide may be collected as shown above because it A. is heavier than air B. is less dense than air C. is insoluble in water D. burns in oxygen to form carbon (IV) oxide |
C |
| 45. |
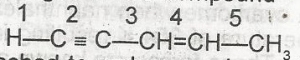 The acidic hydrogen in the compound is the hydrogen attached to carbon number A. 5 B. 4 C. 3 D. 1 |
D |