Year :
1988
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 45 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
An increase in temperature causes an increase in the pressure of a gas because there is an increase in the? A. average velocity of the molecules B. number of collisions between the molecules C. density of the molecules D. free mean path between each molecules and other |
B |
| 32. |
The forces holding naphthalene crystals together can be overcome when naphthalene is heated to a temperature of 354K resulting in the crystal to melting. These forces are known as? A. coulombic B. ionic C. covalent D. Van der waals |
D |
| 33. |
A metallic ion X2+ with an inert gas structure contains 18 electrons. How many protons are there in this ion? A. 20 B. 18 C. 16 D. 2 |
A |
| 34. |
Which of the following physical properties decreases across the Periodic Table? A. Ionization potenial B. Electron affinity C. Electronegativity D. Atomic radius |
D |
| 35. |
What are the possible oxidation numbers of an element if its atomic number is 17? A. -1 and 7 B. -1 and 6 C. -3 and 5 D. -2 and 6 Detailed Solution17 = 2, 8, 7 |
|
| 36. |
The energy changes accompanying the addition of an electron to a gaseous atom is called? A. first ionization energy B. second ionization energy C. electron affinity D. electronegativity |
C |
| 37. |
The molar ratio of oxygen to nitrogen in dissolved air is 2:1 whereas the ratio is 4:1 in atmospheric air because? A. nitrogen is less soluble than oxygen B. oxygen is heavier than nitrogen C. nitrogen has a higher partial pressure in air D. gases are hydrated in water |
A |
| 38. |
An eruption pollution an environment with gas suspected to be H2S, a poisonous gas. A residue team should spray the environment with? A. water B. moist SO2 C. acidified KMnO4 and water D. water, acidified KMnO4 and oxygen |
B |
| 39. |
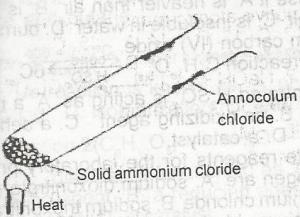 In the experiment above, ammonium chloride crystals deposit on the walls of the tube is as a result of A. evaporation B. recrystallization C. sublimation D. fractional precipitation |
C |
| 40. |
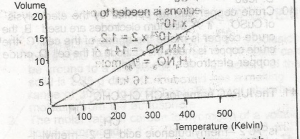 Which of the gas laws does the above graph illustrate? A. Boyle B. Charles C. Graham D. Gay-Lussac |
B |
| 31. |
An increase in temperature causes an increase in the pressure of a gas because there is an increase in the? A. average velocity of the molecules B. number of collisions between the molecules C. density of the molecules D. free mean path between each molecules and other |
B |
| 32. |
The forces holding naphthalene crystals together can be overcome when naphthalene is heated to a temperature of 354K resulting in the crystal to melting. These forces are known as? A. coulombic B. ionic C. covalent D. Van der waals |
D |
| 33. |
A metallic ion X2+ with an inert gas structure contains 18 electrons. How many protons are there in this ion? A. 20 B. 18 C. 16 D. 2 |
A |
| 34. |
Which of the following physical properties decreases across the Periodic Table? A. Ionization potenial B. Electron affinity C. Electronegativity D. Atomic radius |
D |
| 35. |
What are the possible oxidation numbers of an element if its atomic number is 17? A. -1 and 7 B. -1 and 6 C. -3 and 5 D. -2 and 6 Detailed Solution17 = 2, 8, 7 |
| 36. |
The energy changes accompanying the addition of an electron to a gaseous atom is called? A. first ionization energy B. second ionization energy C. electron affinity D. electronegativity |
C |
| 37. |
The molar ratio of oxygen to nitrogen in dissolved air is 2:1 whereas the ratio is 4:1 in atmospheric air because? A. nitrogen is less soluble than oxygen B. oxygen is heavier than nitrogen C. nitrogen has a higher partial pressure in air D. gases are hydrated in water |
A |
| 38. |
An eruption pollution an environment with gas suspected to be H2S, a poisonous gas. A residue team should spray the environment with? A. water B. moist SO2 C. acidified KMnO4 and water D. water, acidified KMnO4 and oxygen |
B |
| 39. |
 In the experiment above, ammonium chloride crystals deposit on the walls of the tube is as a result of A. evaporation B. recrystallization C. sublimation D. fractional precipitation |
C |
| 40. |
 Which of the gas laws does the above graph illustrate? A. Boyle B. Charles C. Graham D. Gay-Lussac |
B |