Year :
1985
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 48 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
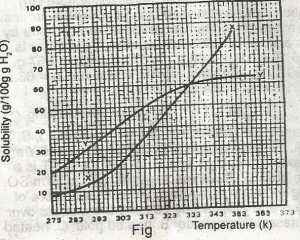 The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is A. 0.2 moles B. 0.7 moles C. 1.5 moles D. 2.0 moles E. 3.0 moles |
B |
| 32. |
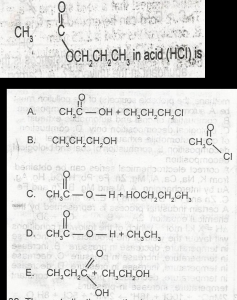 Use the above options to the question. The products formed on hydrolysis of A. A B. B C. C D. D E. E |
C |
| 33. |
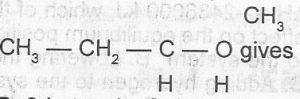 The oxidation of A. 2-butanone B. 2-butanal C. butane D. butanoic acid E. 3-butanal |
A |
| 34. |
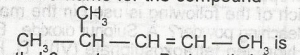 The I.U.P.A.C name for the compound A. 2-methyl-3-pentene B. 4-methyl-2-pentane C. 2-methyl-2-pentene D. 4-methylpent-2-ene E. 2-methyl-3-pentane |
D |
| 35. |
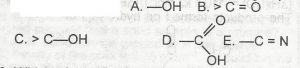 Which of the following is the functional group of carboxylic acids? A. A B. B C. C D. D E. E |
D |
| 36. |
Which of the following conduct electricity? A. sulfur B. graphite C. diamond D. red phosphorus E. yellow phosphorus |
B |
| 37. |
An organic compound contains 72% carbon, 12% hydrogen and 16% oxygen by mass. The empirical formula of the compound is A. C8H22O3 B. C6H10O3 C. C12H12O D. C6H12O E. C3H6O Detailed Solutioncarbon = 72%, hydrogen = 12%, 16%Dividing by the atomic weight we have for C = \(\frac{5}{1}\) = 6 H = \(\frac{12}{1}\) = 12 O = \(\frac{16}{16}\) = 1 Divide by the smallest number, we have = \(\frac{6}{1}\) = 6 H = \(\frac{12}{1}\) = 12 O = \(\frac{1}{1}\) = 1 The formula is C6H12O |
|
| 38. |
0.499g 0f CuSO4.xH2O when heated to constant weight gave a residue of 0.346g. the value of x is? (Cu = 63.5, S = 32.0, O = 16, H = 1) A. 0.5 B. 2.0 C. 3.0 D. 4.0 E. 5.0 |
D |
| 39. |
In the experiment, which of the following observations would suggest that a solid sample is a mixture? The A. solid can be ground to a fine powder B. density of the solid is 2.25gdm-3 C. solid begins to melt at 573k but is not completely melted until 648k D. solid absorbs moisture from the atmosphere and turns into liquid E. solid melts at 300k |
C |
| 40. |
Hydrogen diffuses through a porous plug A. at the same rate as oxygen B. at a slower rate than oxygen C. twice as fast as oxygen D. three times as fast as oxygen E. four times as fast as oxygen |
E |
| 31. |
 The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is A. 0.2 moles B. 0.7 moles C. 1.5 moles D. 2.0 moles E. 3.0 moles |
B |
| 32. |
 Use the above options to the question. The products formed on hydrolysis of A. A B. B C. C D. D E. E |
C |
| 33. |
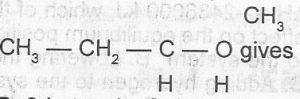 The oxidation of A. 2-butanone B. 2-butanal C. butane D. butanoic acid E. 3-butanal |
A |
| 34. |
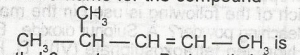 The I.U.P.A.C name for the compound A. 2-methyl-3-pentene B. 4-methyl-2-pentane C. 2-methyl-2-pentene D. 4-methylpent-2-ene E. 2-methyl-3-pentane |
D |
| 35. |
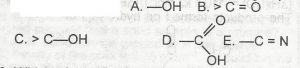 Which of the following is the functional group of carboxylic acids? A. A B. B C. C D. D E. E |
D |
| 36. |
Which of the following conduct electricity? A. sulfur B. graphite C. diamond D. red phosphorus E. yellow phosphorus |
B |
| 37. |
An organic compound contains 72% carbon, 12% hydrogen and 16% oxygen by mass. The empirical formula of the compound is A. C8H22O3 B. C6H10O3 C. C12H12O D. C6H12O E. C3H6O Detailed Solutioncarbon = 72%, hydrogen = 12%, 16%Dividing by the atomic weight we have for C = \(\frac{5}{1}\) = 6 H = \(\frac{12}{1}\) = 12 O = \(\frac{16}{16}\) = 1 Divide by the smallest number, we have = \(\frac{6}{1}\) = 6 H = \(\frac{12}{1}\) = 12 O = \(\frac{1}{1}\) = 1 The formula is C6H12O |
|
| 38. |
0.499g 0f CuSO4.xH2O when heated to constant weight gave a residue of 0.346g. the value of x is? (Cu = 63.5, S = 32.0, O = 16, H = 1) A. 0.5 B. 2.0 C. 3.0 D. 4.0 E. 5.0 |
D |
| 39. |
In the experiment, which of the following observations would suggest that a solid sample is a mixture? The A. solid can be ground to a fine powder B. density of the solid is 2.25gdm-3 C. solid begins to melt at 573k but is not completely melted until 648k D. solid absorbs moisture from the atmosphere and turns into liquid E. solid melts at 300k |
C |
| 40. |
Hydrogen diffuses through a porous plug A. at the same rate as oxygen B. at a slower rate than oxygen C. twice as fast as oxygen D. three times as fast as oxygen E. four times as fast as oxygen |
E |