Year :
1985
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
21 - 30 of 48 Questions
| # | Question | Ans |
|---|---|---|
| 21. |
The atomic number of an element whose cation, X2+, has the ground state electronic configuration 1s22s22p63s22p6 is? A. 16 B. 18 C. 20 D. 22 E. 24 Detailed Solution-The main difference between ground state and excited state is that ground state is a state where electrons in a system are in the lowest possible energy levels whereas excited state is any state of the system that has a higher energy than the ground state.-Calcium atoms have 20 electrons and the shell structure is 2.8. 8.2. The ground state electron configuration of ground state gaseous neutral calcium is [Ar] |
|
| 22. |
When marble is heated to about 1473 K, another white solid is obtained which reacts vigorously with water to give an alkaline solution. The solution contains? A. NaOH B. KOH C. Mg(OH)2 D. Zn(OH)2 E. Ca(OH)2 |
E |
| 23. |
Addition of dilute hydrochloride acid to an aqueous solution of a crystalline salt yield a yellow precipitate and a gas which turned dichromate paper green. The crystalline salt was probably? A. Na2SO4 B. Na2S C. Na2S2O3.5H2O D. Na2CO3 E. NaHCO3 |
C |
| 24. |
The process involved in the conversion of an oil into margarine is known as? A. hydrogenation B. condensation C. pyrolysis D. dehydration E. cracking Detailed SolutionMargarine is an inexpensive alternate to butter, made from oil or a combination of oils through the process of hydrogenation. |
|
| 25. |
An aqueous solution of an inorganic salt gave white precipitate (i) soluble in excess aqueous NaOH (ii) insoluble in excess aqueous NH3 (iii) with dilute HCl. The cation present in the inorganic salt is? A. Na4+ B. Ca++ C. Zn++ D. Al+++ E. Pb++ |
E |
| 26. |
Which of the following roles does sodium chloride play in soap preparation? It A. reacts with glycerol B. purifies the soap C. accelerates the decomposition of the fat or oil D. separates the soap from the glycerol E. converts the fatty acid to its sodium salt |
D |
| 27. |
The function of sulphur during vulcanization rubber is to? A. act as catalyst for the polymerization of rubber molecules B. convert rubber from thermosetting to thermo plastics polymer C. from chains which bind rubber molecules together D. break down rubber polyer E. shorten the chain length of rubber polyer |
C |
| 28. |
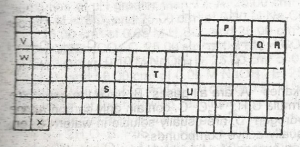 Figure 1 above shows part of the periodic Table which of the elements belongs to the p-block? A. S, T and U B. V, W and X C. S and T only D. P, Q and R E. V, W, X and S |
D |
| 29. |
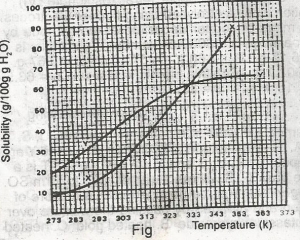 The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. At room temperature (300 K) A. Y is twice as soluble as X B. X is twice as soluble as Y C. X and Y are soluble to the same extent D. X is three times as soluble as Y E. Y is three times as soluble as X |
A |
| 30. |
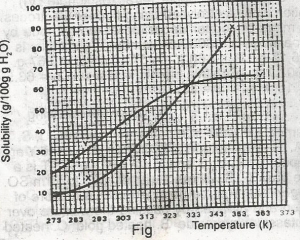 The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If 80 g each of X and Y are taken up in 100 g of water at 353 K we shall have A. only 10g of X undissolved B. only 16 g of Y undissolved C. 10 g of X and 16 g of Y undissolved D. all X and Y dissolved E. all X and Y undissolved Detailed SolutionFor salt X, from the graph, 80g of X dissolves by 349K, so by 353K, all 80g of X is dissolved totally in 100g of water.For salt Y, from the graph, 64g of Y dissolves by 353K, so (80-64)g of Y is left undissolved. This is 16g of Y left undissolved at that temperature. |
| 21. |
The atomic number of an element whose cation, X2+, has the ground state electronic configuration 1s22s22p63s22p6 is? A. 16 B. 18 C. 20 D. 22 E. 24 Detailed Solution-The main difference between ground state and excited state is that ground state is a state where electrons in a system are in the lowest possible energy levels whereas excited state is any state of the system that has a higher energy than the ground state.-Calcium atoms have 20 electrons and the shell structure is 2.8. 8.2. The ground state electron configuration of ground state gaseous neutral calcium is [Ar] |
|
| 22. |
When marble is heated to about 1473 K, another white solid is obtained which reacts vigorously with water to give an alkaline solution. The solution contains? A. NaOH B. KOH C. Mg(OH)2 D. Zn(OH)2 E. Ca(OH)2 |
E |
| 23. |
Addition of dilute hydrochloride acid to an aqueous solution of a crystalline salt yield a yellow precipitate and a gas which turned dichromate paper green. The crystalline salt was probably? A. Na2SO4 B. Na2S C. Na2S2O3.5H2O D. Na2CO3 E. NaHCO3 |
C |
| 24. |
The process involved in the conversion of an oil into margarine is known as? A. hydrogenation B. condensation C. pyrolysis D. dehydration E. cracking Detailed SolutionMargarine is an inexpensive alternate to butter, made from oil or a combination of oils through the process of hydrogenation. |
|
| 25. |
An aqueous solution of an inorganic salt gave white precipitate (i) soluble in excess aqueous NaOH (ii) insoluble in excess aqueous NH3 (iii) with dilute HCl. The cation present in the inorganic salt is? A. Na4+ B. Ca++ C. Zn++ D. Al+++ E. Pb++ |
E |
| 26. |
Which of the following roles does sodium chloride play in soap preparation? It A. reacts with glycerol B. purifies the soap C. accelerates the decomposition of the fat or oil D. separates the soap from the glycerol E. converts the fatty acid to its sodium salt |
D |
| 27. |
The function of sulphur during vulcanization rubber is to? A. act as catalyst for the polymerization of rubber molecules B. convert rubber from thermosetting to thermo plastics polymer C. from chains which bind rubber molecules together D. break down rubber polyer E. shorten the chain length of rubber polyer |
C |
| 28. |
 Figure 1 above shows part of the periodic Table which of the elements belongs to the p-block? A. S, T and U B. V, W and X C. S and T only D. P, Q and R E. V, W, X and S |
D |
| 29. |
 The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. At room temperature (300 K) A. Y is twice as soluble as X B. X is twice as soluble as Y C. X and Y are soluble to the same extent D. X is three times as soluble as Y E. Y is three times as soluble as X |
A |
| 30. |
 The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If 80 g each of X and Y are taken up in 100 g of water at 353 K we shall have A. only 10g of X undissolved B. only 16 g of Y undissolved C. 10 g of X and 16 g of Y undissolved D. all X and Y dissolved E. all X and Y undissolved Detailed SolutionFor salt X, from the graph, 80g of X dissolves by 349K, so by 353K, all 80g of X is dissolved totally in 100g of water.For salt Y, from the graph, 64g of Y dissolves by 353K, so (80-64)g of Y is left undissolved. This is 16g of Y left undissolved at that temperature. |