Year :
1984
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 45 of 45 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
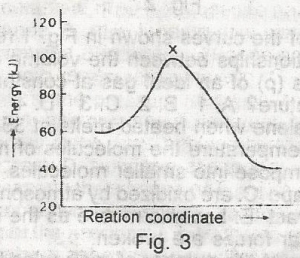 The diagram above (Fig.3) shows the energy profile for the reaction A + B + = C + D From this diagram, it is clear that the reaction is A. spontaneous B. isothermal C. adiabatic D. exothermic E. endothermic |
D |
| 42. |
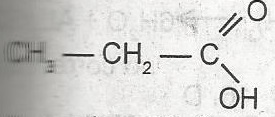 The name of the compound above is A. acetic acid B. propanal C. propanol D. ethanoic acid E. propanoic acid |
E |
| 43. |
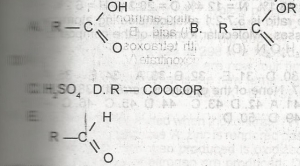 USE THE DIAGRAM ABOVE TO ANSWER THIS QUESTION. A. A B. B C. C D. D E. E |
A |
| 44. |
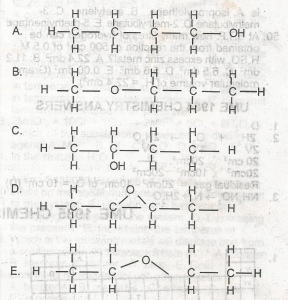 Which of the following structural formula is NOT isomeric with the others? A. A B. B C. C D. D E. E |
D |
| 45. |
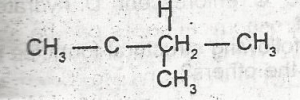 The I.U.P.A.C name for the compound is A. isopropylethene B. acetylene C. 3- methylbutane D. 2-methylbutane E. 5-methylpentane |
D |
| 41. |
 The diagram above (Fig.3) shows the energy profile for the reaction A + B + = C + D From this diagram, it is clear that the reaction is A. spontaneous B. isothermal C. adiabatic D. exothermic E. endothermic |
D |
| 42. |
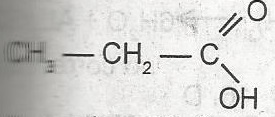 The name of the compound above is A. acetic acid B. propanal C. propanol D. ethanoic acid E. propanoic acid |
E |
| 43. |
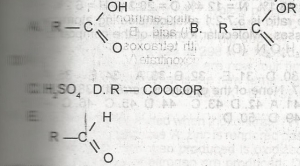 USE THE DIAGRAM ABOVE TO ANSWER THIS QUESTION. A. A B. B C. C D. D E. E |
A |
| 44. |
 Which of the following structural formula is NOT isomeric with the others? A. A B. B C. C D. D E. E |
D |
| 45. |
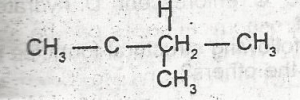 The I.U.P.A.C name for the compound is A. isopropylethene B. acetylene C. 3- methylbutane D. 2-methylbutane E. 5-methylpentane |
D |