Year :
1984
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 45 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
Sodium hydroxide (NaOH) pellets are A. deliquescent B. hygroscopic C. efflorescent D. hydrated E. fluorescent |
A |
| 32. |
Alkanes A. are all gases B. have the general formula CnH2n + 2O C. contain only carbon and hydrogen D. are usually soluble in water E. are usually active compounds |
C |
| 33. |
If an excess of a liquid hydrocarbon is poured into a jar of chlorine, and the sealed jar is then exposed for several hours to bring sunlight, all the chlorine gas is consumed. The hydrocarbon is said to have undergone A. a polymerization reaction B. an isomerization reaction C. an addition reaction D. a substitution reaction E. a reduction reaction |
D |
| 34. |
The function of conc. H2SO4 in the esterification of ethanoic acid with ethanol is to A. serve as a dehydrating agent B. serve as a solvent C. act as a catalyst D. prevent any side reaction E. serve as an oxidizing agent |
C |
| 35. |
A piece of sea shell, when dropped into a dilute solution of hydrochloric acid, produces a colourless, odourless gas which turns clear limewater milky. The sea shell contains A. sodium chloride B. ammonium nitrate C. calcium carbonate D. calcium chloride E. magnesium chloride |
C |
| 36. |
Which of the following electrodes is used in electrolysis of bauxite (Al2O3) in molten cryolite? A. Steel electrodes B. Carbon electrodes C. Mercury electrodes D. Carbon anode and steel cathode E. Carbon cathode and stell anode |
B |
| 37. |
An aqueous solution of a metal salt, M, gives a white precipitate with NaOH which dissolves in excess NaOH. With aqueous ammonia, the solution of M also gives a white precipitate which dissolves in excess ammonia. Therefore cartion in M is A. Zn ++ B. Ca++ C. Ai+++ D. Pb++ E. Cu++ |
A |
| 38. |
At S. T. P how many litres of hydrogen can be obtained from the reaction of 500 cm3 of 0.5 M H 2SO 4 with excess zinc metal? A. 22.4 dm 3 B. 2 dm 3 C. 5 dm 3 D. 6 dm 3 E. 00 dm 3 |
D |
| 39. |
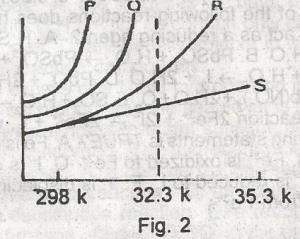 The solubility curves of four substances are shown in the figure above. Which of the four substances would crystallize from a saturated solution cooled from 353K(80oC)to 323K(50C)? A. P and Q B. P and R C. P and S D. R and S E. Q and R |
D |
| 40. |
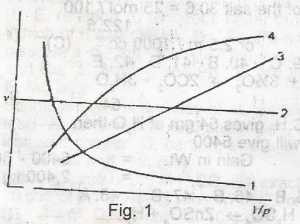 Which of the curves shown in the figure given represents the relationships between the volume(V) and pressure(P) of an ideal gas at constant temperature A. 1 B. 2 C. 3 D. 4 E. 1 and 3 |
C |
| 31. |
Sodium hydroxide (NaOH) pellets are A. deliquescent B. hygroscopic C. efflorescent D. hydrated E. fluorescent |
A |
| 32. |
Alkanes A. are all gases B. have the general formula CnH2n + 2O C. contain only carbon and hydrogen D. are usually soluble in water E. are usually active compounds |
C |
| 33. |
If an excess of a liquid hydrocarbon is poured into a jar of chlorine, and the sealed jar is then exposed for several hours to bring sunlight, all the chlorine gas is consumed. The hydrocarbon is said to have undergone A. a polymerization reaction B. an isomerization reaction C. an addition reaction D. a substitution reaction E. a reduction reaction |
D |
| 34. |
The function of conc. H2SO4 in the esterification of ethanoic acid with ethanol is to A. serve as a dehydrating agent B. serve as a solvent C. act as a catalyst D. prevent any side reaction E. serve as an oxidizing agent |
C |
| 35. |
A piece of sea shell, when dropped into a dilute solution of hydrochloric acid, produces a colourless, odourless gas which turns clear limewater milky. The sea shell contains A. sodium chloride B. ammonium nitrate C. calcium carbonate D. calcium chloride E. magnesium chloride |
C |
| 36. |
Which of the following electrodes is used in electrolysis of bauxite (Al2O3) in molten cryolite? A. Steel electrodes B. Carbon electrodes C. Mercury electrodes D. Carbon anode and steel cathode E. Carbon cathode and stell anode |
B |
| 37. |
An aqueous solution of a metal salt, M, gives a white precipitate with NaOH which dissolves in excess NaOH. With aqueous ammonia, the solution of M also gives a white precipitate which dissolves in excess ammonia. Therefore cartion in M is A. Zn ++ B. Ca++ C. Ai+++ D. Pb++ E. Cu++ |
A |
| 38. |
At S. T. P how many litres of hydrogen can be obtained from the reaction of 500 cm3 of 0.5 M H 2SO 4 with excess zinc metal? A. 22.4 dm 3 B. 2 dm 3 C. 5 dm 3 D. 6 dm 3 E. 00 dm 3 |
D |
| 39. |
 The solubility curves of four substances are shown in the figure above. Which of the four substances would crystallize from a saturated solution cooled from 353K(80oC)to 323K(50C)? A. P and Q B. P and R C. P and S D. R and S E. Q and R |
D |
| 40. |
 Which of the curves shown in the figure given represents the relationships between the volume(V) and pressure(P) of an ideal gas at constant temperature A. 1 B. 2 C. 3 D. 4 E. 1 and 3 |
C |