Year :
1994
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 50 of 52 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
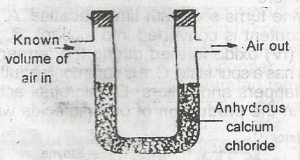 The set-up above would be useful for determining the amount of A. oxygen in air B. water vapour in air C. CO2 in air D. arygen in air |
B |
| 42. |
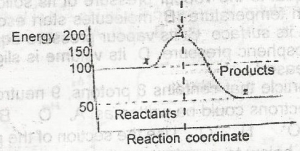 The ∆H for the reaction represented by the energy profile above is A. -100 kJ mol--1 B. + 100 kJ mol-1 C. +50 kJ mol-1 D. -50 kJ mol-1 |
D |
| 43. |
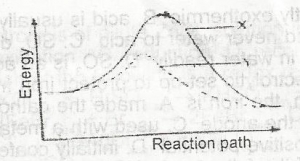 In the diagram above the activation energy is represented by A. y - x B. x C. x - z D. y |
A |
| 44. |
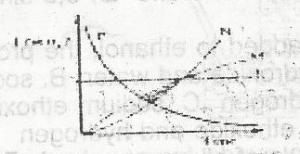 2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from the reaction above, which of the following curves represents the consumption of calcium trioxocarbonate (IV) as dilute HCL is added to it? A. L B. M C. N D. P |
B |
| 45. |
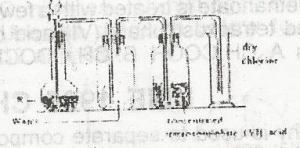 In the diagram above, R is a mixture of A. potassium tetraxochlorate (VII) and concentrated H2SO4 B. potassium trioxochlorate (V) and concentrated H2SO4 C. potassium tetratoxomanganate (VII) and concentrated HCI D. manganes (IV) oxide and concentrated HCI |
D |
| 46. |
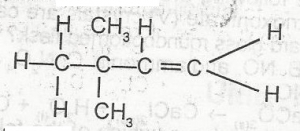 The IUPAC name of the compound above is A. 2,2-dimethyl but -1-yne B. 2,2-dmethyl but -1-ene C. 3,3-dimethyl but -1-ene D. 3,3-dimethyl but-1-yne |
C |
| 47. |
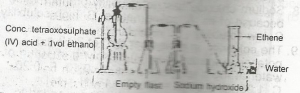 The reaction taking place in flask G is known as A. hydrolysis B. double decomposition C. dehydration D. pyrolysis |
C |
| 48. |
MnO4(aq) + 8H + 5Fe2+ \(\to\) Mn2+(aq) + 5Fe3+(aq) + H2O. The oxidation number of maganese in the above reaction changed from A. +7 to +2 B. +6 to +2 C. +5 to +2 D. +4 to +2 Detailed Solution\(MnO_{{4}{(aq)}}\) has a charge of \(-1\)Let the oxidation number of \(Mn\) in \(MnO_{{4}{(aq)}}\) be X Recall, the oxidation number of \(O_{2}\) is \(-2\) \(\therefore\) \(X + (-2\times 4) = -1\) \(X - 8 = -1\) \(X = +7\) |
|
| 49. |
Which of these metals CANNOT replace hydrogen from alkaline solutions? A. Aluminium B. Zinc C. Tin D. Iron |
A |
| 50. |
Clothes should be properly rinsed with water after bleaching because A. the bleach decolourizes the clothes B. chlorine reacts with fabrics during bleaching C. the clothes are sterilized during bleaching D. hydrogen chloride solution is produced during bleaching |
D |
| 41. |
 The set-up above would be useful for determining the amount of A. oxygen in air B. water vapour in air C. CO2 in air D. arygen in air |
B |
| 42. |
 The ∆H for the reaction represented by the energy profile above is A. -100 kJ mol--1 B. + 100 kJ mol-1 C. +50 kJ mol-1 D. -50 kJ mol-1 |
D |
| 43. |
 In the diagram above the activation energy is represented by A. y - x B. x C. x - z D. y |
A |
| 44. |
 2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from the reaction above, which of the following curves represents the consumption of calcium trioxocarbonate (IV) as dilute HCL is added to it? A. L B. M C. N D. P |
B |
| 45. |
 In the diagram above, R is a mixture of A. potassium tetraxochlorate (VII) and concentrated H2SO4 B. potassium trioxochlorate (V) and concentrated H2SO4 C. potassium tetratoxomanganate (VII) and concentrated HCI D. manganes (IV) oxide and concentrated HCI |
D |
| 46. |
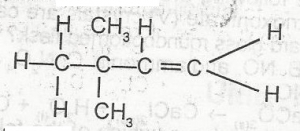 The IUPAC name of the compound above is A. 2,2-dimethyl but -1-yne B. 2,2-dmethyl but -1-ene C. 3,3-dimethyl but -1-ene D. 3,3-dimethyl but-1-yne |
C |
| 47. |
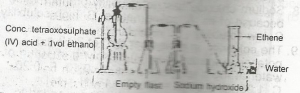 The reaction taking place in flask G is known as A. hydrolysis B. double decomposition C. dehydration D. pyrolysis |
C |
| 48. |
MnO4(aq) + 8H + 5Fe2+ \(\to\) Mn2+(aq) + 5Fe3+(aq) + H2O. The oxidation number of maganese in the above reaction changed from A. +7 to +2 B. +6 to +2 C. +5 to +2 D. +4 to +2 Detailed Solution\(MnO_{{4}{(aq)}}\) has a charge of \(-1\)Let the oxidation number of \(Mn\) in \(MnO_{{4}{(aq)}}\) be X Recall, the oxidation number of \(O_{2}\) is \(-2\) \(\therefore\) \(X + (-2\times 4) = -1\) \(X - 8 = -1\) \(X = +7\) |
|
| 49. |
Which of these metals CANNOT replace hydrogen from alkaline solutions? A. Aluminium B. Zinc C. Tin D. Iron |
A |
| 50. |
Clothes should be properly rinsed with water after bleaching because A. the bleach decolourizes the clothes B. chlorine reacts with fabrics during bleaching C. the clothes are sterilized during bleaching D. hydrogen chloride solution is produced during bleaching |
D |