Year :
1994
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 52 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
SO3 is NOT directly dissolved in water in the preparation of H2SO4 by the contact process because? A. the reaction between SO3 and water is violently exothermic B. acid is usually added to water and never water to acid C. SO3 does not dissolve in water readily D. SO3 is an acid gas |
A |
| 32. |
In an electrolytic set-up to protect iron from corrosion, the iron is? A. made cathode B. made anode C. used with a metal of lower electropositive potential D. initially coated with tin Detailed SolutionIn cathodic protection,iron object is made cathode by connecting it with reactive elements like magnesium,zinc. |
|
| 33. |
Which of the following is NOT true of metals? A. They are good conductors of electricity B. They ionize by electron loss C. Their oxides are acidic D. They have high melting points |
C |
| 34. |
Which of the following is the correct order of decreasing activity of the metals Fe, Ca, Al and Na? A. Fe> Ca> Al> Na B. Na> Ca> Al> Fe C. Al> Fe> Na> Ca D. Ca> Na> Fe>, Al |
B |
| 35. |
Which of the following compounds is NOT an isomers of 2,2 dimethylbutane? A. 2-methylbutane B. 3-methylpentane C. 2,3-dimethylbutane D. 2-methylpentane |
A |
| 36. |
When sodium is added to ethanol, the products are? A. sodium hydroxide and water B. sodium hydroxide and hydrogen C. sodium ethoxide and water D. sodium ethoxide and hydrogen |
D |
| 37. |
The general formula of alkanoates is? A. RCHO B. R2CO C. RCOOH D. RCOOR Detailed SolutionOtherwise known as ESTERS, they are easily gotten from a reaction between the CARBOXYLIC acids and ALCOHOLSRCOOH + R'OH yields a combination of an ester(RCOOR') and water. |
|
| 38. |
One mole of a hydrocarbon contains 48 g of carbon. If it vapour density is 28, the hydrocarbon is? A. an alkane B. an alkene C. an alkyne D. aromatic (C = 12, H = 1) |
B |
| 39. |
Which of the following reagents will confirm the presence of unsaturation in a compound? A. Fehling's solution B. Bromine water C. Tollen's reagent D. Benedict's solution |
B |
| 40. |
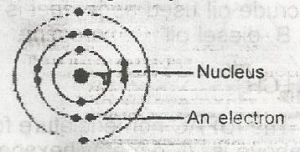 The diagram above represents an atom of A. magnesium B. helium C. chlorine D. neon Detailed SolutionThe diagram refers to an atom with electronic configuration 2,8,2 hence the atomic number = 12. From the periodic table, we have the atom to be Mg (Magnesium). |
| 31. |
SO3 is NOT directly dissolved in water in the preparation of H2SO4 by the contact process because? A. the reaction between SO3 and water is violently exothermic B. acid is usually added to water and never water to acid C. SO3 does not dissolve in water readily D. SO3 is an acid gas |
A |
| 32. |
In an electrolytic set-up to protect iron from corrosion, the iron is? A. made cathode B. made anode C. used with a metal of lower electropositive potential D. initially coated with tin Detailed SolutionIn cathodic protection,iron object is made cathode by connecting it with reactive elements like magnesium,zinc. |
|
| 33. |
Which of the following is NOT true of metals? A. They are good conductors of electricity B. They ionize by electron loss C. Their oxides are acidic D. They have high melting points |
C |
| 34. |
Which of the following is the correct order of decreasing activity of the metals Fe, Ca, Al and Na? A. Fe> Ca> Al> Na B. Na> Ca> Al> Fe C. Al> Fe> Na> Ca D. Ca> Na> Fe>, Al |
B |
| 35. |
Which of the following compounds is NOT an isomers of 2,2 dimethylbutane? A. 2-methylbutane B. 3-methylpentane C. 2,3-dimethylbutane D. 2-methylpentane |
A |
| 36. |
When sodium is added to ethanol, the products are? A. sodium hydroxide and water B. sodium hydroxide and hydrogen C. sodium ethoxide and water D. sodium ethoxide and hydrogen |
D |
| 37. |
The general formula of alkanoates is? A. RCHO B. R2CO C. RCOOH D. RCOOR Detailed SolutionOtherwise known as ESTERS, they are easily gotten from a reaction between the CARBOXYLIC acids and ALCOHOLSRCOOH + R'OH yields a combination of an ester(RCOOR') and water. |
|
| 38. |
One mole of a hydrocarbon contains 48 g of carbon. If it vapour density is 28, the hydrocarbon is? A. an alkane B. an alkene C. an alkyne D. aromatic (C = 12, H = 1) |
B |
| 39. |
Which of the following reagents will confirm the presence of unsaturation in a compound? A. Fehling's solution B. Bromine water C. Tollen's reagent D. Benedict's solution |
B |
| 40. |
 The diagram above represents an atom of A. magnesium B. helium C. chlorine D. neon Detailed SolutionThe diagram refers to an atom with electronic configuration 2,8,2 hence the atomic number = 12. From the periodic table, we have the atom to be Mg (Magnesium). |