Year :
2017
Title :
Chemistry
Exam :
WASSCE/WAEC MAY/JUNE
Paper 1 | Objectives
41 - 50 of 50 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
Calculate the mass of copper deposited if a current of 0.45A flows through \(CuSO_{4}\) solution for 1hour 15mins. [Cu=64.0, S= 32.0, O =16.0, 1F= 96500C] A. 6.40g B. 0.67g C. 0.64g D. 0.45g Detailed Solution\(Cu^{2+} + 2e^{-} \to Cu_{(s)}\)\( 2 \times 96500C = 193000C deposits 64g of Cu\) \(M \propto It\) \(Q = It = 0.45A \times 1hr15mins\) = \( 0.45 \times 75 \times 60\) [converting the time to seconds] Quantity of electricity passed = 2025C \(193000C \to 64g Cu\) \(1C \to \frac{64}{193000}\) \(2025C \to \frac{64}{193000} \times 2025\) \(= 0.6715g \approxeq 0.67g\) |
|
| 42. |
Classification of alkanols is based on the A. number of carbon atoms present in the compound B. molecular mass of the compound C. molecular formula of the compound D. number of alkyl groups bonded to the carbon having the hydroxyl group Detailed SolutionAlkanols can be classified as primary, secondary or tertiary, according to the number of alkyl groups attached to the carbon which bears the -OH group. |
|
| 43. |
The name of the compound \(C_{4}H_{9}COOC_{3}H_{7}\) is A. propyl pentanoate B. pentyl propanoate C. butyl propanoate D. propyl butanoate Detailed SolutionThe alkyl group is attached to the parent chain being a carboxylic acid. The name of the compound is gotten by writing the name of the attached alkyl group first and then the parent carboxylic acid is written but you change the -oic acid to -oate.The alkyl group in this case is \(C_{3}H_{7}\) The parent carboxylic acid is \(C_{4}H_{9}COOH\) which is pentanoic acid. Hence, the name is propyl pentanoate. |
|
| 44. |
Which of the following pairs of gases are pollutants from car exhaust? A. Nitrogen(II) oxide and Carbon(IV) oxide B. Carbon(IV) oxide and Nitrogen C. Carbon(II) oxide and Sulphur(IV) oxide D. Carbon(II) oxide and oxygen |
C |
| 45. |
Fats and oils can be obtained from any of the following sources except A. paraffin oil B. animals C. groundnuts D. palm kernel Detailed SolutionParaffin oil is gotten from petroleum distillation and can't be a source of fats and oil. |
|
| 46. |
The condensation of two units of glucose will produce a dissacharide with the formula \(C_{12}H_{22}O_{11}\) which is A. sucrose B. maltose C. galactose D. mannose Detailed SolutionA maltose is a dissacharide formed from two units of glucose. |
|
| 47. |
The IUPAC name for the compound \(ClCH_{2}CH_{2}OH\) is A. 1- chloropropanol B. 2-chloropropanol C. 1-chloroethanol D. 2-chloroethanol Detailed SolutionThe parent chain for the compound is \(C_{2}H_{5}OH\) in which one of the hydrogen atoms is replaced by the Cl atom. Giving the chlorine the smallest number while naming, we have 1- chloroethanol. |
|
| 48. |
The real harmful effect of the release of chlorofluorocarbons into the atmosphere is that it eventually causes A. excessive ultraviolet light from the sun to reach the earth surface B. excessive release of infrared light from the sun to the earth surface C. corrosive effect of the chemicals on humans and animals D. acidic effect of chemicals on humans Detailed SolutionChlorofluorocarbons are harmful to environment and living beings because it increases greenhouse gases in the environment which results in forming holes in the ozone layer.. And if this happens it will cause to an increase in the ultraviolet rays exposer, and which will make the earth's surface much hotter than usual. |
|
| 49. |
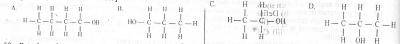 A primary alkanol has a molecular mass of 60. What is the structural formula of the compound? [C= 12.0, H= 1.0, O= 16.0) A. A B. B C. C D. D Detailed SolutionAlkanols have the formula \(C_{n}H_{2n+1}OH\).⇒ \( 12n + (1\times (2n+1)) + 16.0 + 1.0 = 60\) = (14n +18 = 60\) \(n = \frac{60-18}{14} = \frac{42}{14} = 3\) Hence, the alkanol is gotten by putting n=3, =\(C_{3}H_{7}OH\) |
|
| 50. |
Petroleum is a non-renewable source of energy because it A. is formed naturally B. is cheap C. can be recycled after use D. cannot be regenerated once used up |
D |
| 41. |
Calculate the mass of copper deposited if a current of 0.45A flows through \(CuSO_{4}\) solution for 1hour 15mins. [Cu=64.0, S= 32.0, O =16.0, 1F= 96500C] A. 6.40g B. 0.67g C. 0.64g D. 0.45g Detailed Solution\(Cu^{2+} + 2e^{-} \to Cu_{(s)}\)\( 2 \times 96500C = 193000C deposits 64g of Cu\) \(M \propto It\) \(Q = It = 0.45A \times 1hr15mins\) = \( 0.45 \times 75 \times 60\) [converting the time to seconds] Quantity of electricity passed = 2025C \(193000C \to 64g Cu\) \(1C \to \frac{64}{193000}\) \(2025C \to \frac{64}{193000} \times 2025\) \(= 0.6715g \approxeq 0.67g\) |
|
| 42. |
Classification of alkanols is based on the A. number of carbon atoms present in the compound B. molecular mass of the compound C. molecular formula of the compound D. number of alkyl groups bonded to the carbon having the hydroxyl group Detailed SolutionAlkanols can be classified as primary, secondary or tertiary, according to the number of alkyl groups attached to the carbon which bears the -OH group. |
|
| 43. |
The name of the compound \(C_{4}H_{9}COOC_{3}H_{7}\) is A. propyl pentanoate B. pentyl propanoate C. butyl propanoate D. propyl butanoate Detailed SolutionThe alkyl group is attached to the parent chain being a carboxylic acid. The name of the compound is gotten by writing the name of the attached alkyl group first and then the parent carboxylic acid is written but you change the -oic acid to -oate.The alkyl group in this case is \(C_{3}H_{7}\) The parent carboxylic acid is \(C_{4}H_{9}COOH\) which is pentanoic acid. Hence, the name is propyl pentanoate. |
|
| 44. |
Which of the following pairs of gases are pollutants from car exhaust? A. Nitrogen(II) oxide and Carbon(IV) oxide B. Carbon(IV) oxide and Nitrogen C. Carbon(II) oxide and Sulphur(IV) oxide D. Carbon(II) oxide and oxygen |
C |
| 45. |
Fats and oils can be obtained from any of the following sources except A. paraffin oil B. animals C. groundnuts D. palm kernel Detailed SolutionParaffin oil is gotten from petroleum distillation and can't be a source of fats and oil. |
| 46. |
The condensation of two units of glucose will produce a dissacharide with the formula \(C_{12}H_{22}O_{11}\) which is A. sucrose B. maltose C. galactose D. mannose Detailed SolutionA maltose is a dissacharide formed from two units of glucose. |
|
| 47. |
The IUPAC name for the compound \(ClCH_{2}CH_{2}OH\) is A. 1- chloropropanol B. 2-chloropropanol C. 1-chloroethanol D. 2-chloroethanol Detailed SolutionThe parent chain for the compound is \(C_{2}H_{5}OH\) in which one of the hydrogen atoms is replaced by the Cl atom. Giving the chlorine the smallest number while naming, we have 1- chloroethanol. |
|
| 48. |
The real harmful effect of the release of chlorofluorocarbons into the atmosphere is that it eventually causes A. excessive ultraviolet light from the sun to reach the earth surface B. excessive release of infrared light from the sun to the earth surface C. corrosive effect of the chemicals on humans and animals D. acidic effect of chemicals on humans Detailed SolutionChlorofluorocarbons are harmful to environment and living beings because it increases greenhouse gases in the environment which results in forming holes in the ozone layer.. And if this happens it will cause to an increase in the ultraviolet rays exposer, and which will make the earth's surface much hotter than usual. |
|
| 49. |
 A primary alkanol has a molecular mass of 60. What is the structural formula of the compound? [C= 12.0, H= 1.0, O= 16.0) A. A B. B C. C D. D Detailed SolutionAlkanols have the formula \(C_{n}H_{2n+1}OH\).⇒ \( 12n + (1\times (2n+1)) + 16.0 + 1.0 = 60\) = (14n +18 = 60\) \(n = \frac{60-18}{14} = \frac{42}{14} = 3\) Hence, the alkanol is gotten by putting n=3, =\(C_{3}H_{7}OH\) |
|
| 50. |
Petroleum is a non-renewable source of energy because it A. is formed naturally B. is cheap C. can be recycled after use D. cannot be regenerated once used up |
D |