Year :
1987
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 34 of 34 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
An element Z, contained 90% of 168Z and 10% of Z1818 its relative atomic mass is? A. 16.0 B. 16.2 C. 17.0 D. 17.8 Detailed SolutionMass number of Z = 16 while that of Z is 1890 x 16 = 14.4 10 x 18 = 1.8 100 1 100 1 it relative atomic mass = 14.4 + 1.8 = 162 |
|
| 32. |
 Which is the correct set of results for tests conducted respectively on fresh lime juice and ethanol. A. A B. B C. C D. D |
A |
| 33. |
 The times taken for iodine to be liberated in the reaction between sodium thiosulphate and hydrochloric acid at various temperature are as follows. These results suggest that A. for a 10o rise in temperature, rate of reaction is double B. for a 10 o rise in temperature, rate of reaction is halved C. time taken for iodine to appear does not depend on temperature D. for a 10 o rise in temperature rate of reaction is tripled |
A |
| 34. |
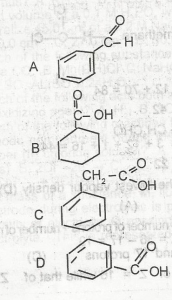 The structure of benzoic acid is A. A B. B C. C D. D |
D |
| 31. |
An element Z, contained 90% of 168Z and 10% of Z1818 its relative atomic mass is? A. 16.0 B. 16.2 C. 17.0 D. 17.8 Detailed SolutionMass number of Z = 16 while that of Z is 1890 x 16 = 14.4 10 x 18 = 1.8 100 1 100 1 it relative atomic mass = 14.4 + 1.8 = 162 |
|
| 32. |
 Which is the correct set of results for tests conducted respectively on fresh lime juice and ethanol. A. A B. B C. C D. D |
A |
| 33. |
 The times taken for iodine to be liberated in the reaction between sodium thiosulphate and hydrochloric acid at various temperature are as follows. These results suggest that A. for a 10o rise in temperature, rate of reaction is double B. for a 10 o rise in temperature, rate of reaction is halved C. time taken for iodine to appear does not depend on temperature D. for a 10 o rise in temperature rate of reaction is tripled |
A |
| 34. |
 The structure of benzoic acid is A. A B. B C. C D. D |
D |