Year :
1980
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 47 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
If one of the following oxides is heated with hydrogen or carbon using a bunsen burner, it is not reduced to the metal. Which one is it? A. Lead oxide B. Magnesium oxide C. Copper oxide D. Tin oxide E. Iron oxide |
B |
| 32. |
Which of the following statement is false? A. Acids react with some metals, liberating hydrogen B. Acids react with carbonates, liberating carbondioxide C. Acids conduct electricity and are decomposed by the current, liberating hydrogen at the anode D. Acids have a sour taste E. Acids change the colour of indicators |
C |
| 33. |
A mixture of ethanol, sodium dichromate, and water is allowed to drop gradually into a boiling solution containing one part concentrated H2SO A. ethanoic acid B. acetaldehyde C. ethyl acetate D. methanol E. ethylene |
A |
| 34. |
When chlorine gas is passed into a solution of sulphur dioxide? A. sulphur dioxide is reduced to sulphur B. the chlorine is oxidized to hydrochloric acid C. dilute sulphuric acid and hydrochloric acid are produced D. Hydrochloric acid and sulphur are produced E. sulphur trioxide is the only product |
C |
| 35. |
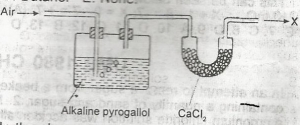 In the above experiment X is A. pure nitrogen B. a mixture of nitrogen and oxygen C. a mixture of nitrogen and carbondioxide D. a mixture of oxygen and inert gases E. a mixture of nitrogen and inert gases |
E |
| 36. |
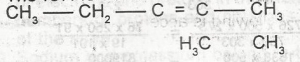 The IUPAC name for the compound is A. 2,3-dimethylpent-2,3-ene B. 2, 3- dimethylpent-2-ene C. 3, 4-dimethylpent-3-ene D. 3,4-dimethylpentene E. 3,4-dimethlhept-2-ene |
B |
| 37. |
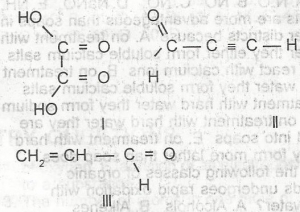 Which of the following compounds will take up the molecules of bromine? A. l B. ll C. lll D. l and ll E. l and lll |
B |
| 38. |
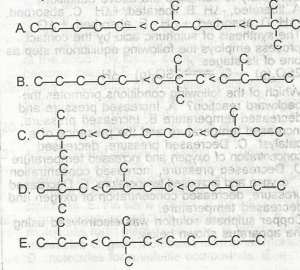 Which of the following is the correct order in the above diagram increasing boiling point, of the isomeric \(C_{5}H_{12}\) compounds? A. A B. B C. C D. D E. E Detailed SolutionThe boiling points of each of the compounds:Pentane - 36.1°C ; 2- methylbutane - 27.8°C ; dimethylpropane - 10°C. Therefore, the order of boiling point in ascending order is Dimethylpropane < 2-methylbutane < pentane. |
|
| 39. |
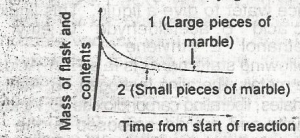 The following graph demonstrates the rate of reaction between calcium carbonate (marble)and dilute hydrochloric acid. The graph shows that the rate of reaction is initially greatest when A. the initial slope of the curve is steepest and this occur when small pieces of marble are used and is due to a small surface area of reactant B. the occur when large pieces of marble are used and is due to a small surface area of reactant C. the initial slope of the curve is steepest and this occurs when small pieces of marble are used and is due to a large surface area of reactant D. the initial slope of the curve is steepest and this occur when large surface area of reactant E. the initial slope of the curve is least steep and this occur when small pieces of marble are used and is due to the increased surface area of the reactant |
C |
| 40. |
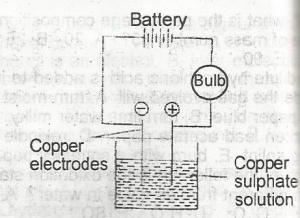 Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed? A. The bulb lights and copper is deposited at both electrodes B. the bulb lights and copper is deposited at the anode and disappears from the cathode C. the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode D. the bulb lights and copper is deposited at the cathode and disappears from the anode E. the bulb lights but no additional changes are observed |
D |
| 31. |
If one of the following oxides is heated with hydrogen or carbon using a bunsen burner, it is not reduced to the metal. Which one is it? A. Lead oxide B. Magnesium oxide C. Copper oxide D. Tin oxide E. Iron oxide |
B |
| 32. |
Which of the following statement is false? A. Acids react with some metals, liberating hydrogen B. Acids react with carbonates, liberating carbondioxide C. Acids conduct electricity and are decomposed by the current, liberating hydrogen at the anode D. Acids have a sour taste E. Acids change the colour of indicators |
C |
| 33. |
A mixture of ethanol, sodium dichromate, and water is allowed to drop gradually into a boiling solution containing one part concentrated H2SO A. ethanoic acid B. acetaldehyde C. ethyl acetate D. methanol E. ethylene |
A |
| 34. |
When chlorine gas is passed into a solution of sulphur dioxide? A. sulphur dioxide is reduced to sulphur B. the chlorine is oxidized to hydrochloric acid C. dilute sulphuric acid and hydrochloric acid are produced D. Hydrochloric acid and sulphur are produced E. sulphur trioxide is the only product |
C |
| 35. |
 In the above experiment X is A. pure nitrogen B. a mixture of nitrogen and oxygen C. a mixture of nitrogen and carbondioxide D. a mixture of oxygen and inert gases E. a mixture of nitrogen and inert gases |
E |
| 36. |
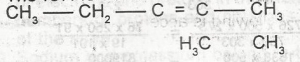 The IUPAC name for the compound is A. 2,3-dimethylpent-2,3-ene B. 2, 3- dimethylpent-2-ene C. 3, 4-dimethylpent-3-ene D. 3,4-dimethylpentene E. 3,4-dimethlhept-2-ene |
B |
| 37. |
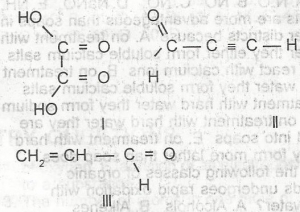 Which of the following compounds will take up the molecules of bromine? A. l B. ll C. lll D. l and ll E. l and lll |
B |
| 38. |
 Which of the following is the correct order in the above diagram increasing boiling point, of the isomeric \(C_{5}H_{12}\) compounds? A. A B. B C. C D. D E. E Detailed SolutionThe boiling points of each of the compounds:Pentane - 36.1°C ; 2- methylbutane - 27.8°C ; dimethylpropane - 10°C. Therefore, the order of boiling point in ascending order is Dimethylpropane < 2-methylbutane < pentane. |
|
| 39. |
 The following graph demonstrates the rate of reaction between calcium carbonate (marble)and dilute hydrochloric acid. The graph shows that the rate of reaction is initially greatest when A. the initial slope of the curve is steepest and this occur when small pieces of marble are used and is due to a small surface area of reactant B. the occur when large pieces of marble are used and is due to a small surface area of reactant C. the initial slope of the curve is steepest and this occurs when small pieces of marble are used and is due to a large surface area of reactant D. the initial slope of the curve is steepest and this occur when large surface area of reactant E. the initial slope of the curve is least steep and this occur when small pieces of marble are used and is due to the increased surface area of the reactant |
C |
| 40. |
 Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed? A. The bulb lights and copper is deposited at both electrodes B. the bulb lights and copper is deposited at the anode and disappears from the cathode C. the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode D. the bulb lights and copper is deposited at the cathode and disappears from the anode E. the bulb lights but no additional changes are observed |
D |