Year :
2001
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 50 of 50 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
The gas that can best be collected by downward displacement of air is A. Chlorine B. Sulphur (IV) oxide C. Ammonia D. Carbon (IV) oxide |
C |
| 42. |
Which of the following metals burn with brick red flame? A. Pb B. Ca C. Na D. Mg |
B |
| 43. |
Which of the following represent hybridization in ethyne? A. Sp2 B. Sp2d C. Sp3 D. Sp |
D |
| 44. |
C12H22O11(s) + H2SO4(aq) 12C(s) + 11H2O(l) + H2SO4(aq) A. A dehydrate agent B. An oxidizing agent C. A reducing agent D. A catalyst |
A |
| 45. |
When sodium reacts with water, the resulting solution is A. Weakly acidic B. Neutral C. Acidic D. Alkaline |
D |
| 46. |
Which of the following gases contains the least number of atoms at s.t.p? A. 1 mole of butane B. 3 mole of ozone C. 4 mole of chlorine D. 7 mole of argon |
A |
| 47. |
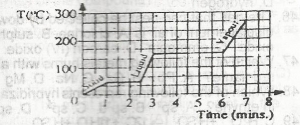 If the gas is cooled, at what temperature will it start to condense? A. 125 oC B. 150oC C. 175oC D. 250oC |
B |
| 48. |
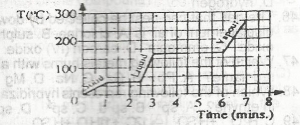 How long does it take all the solid to melt?` A. 2.5 mins B. 6.0 mins C. 1.0 min D. 3.0 mins |
A |
| 49. |
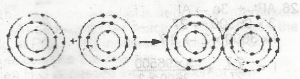 The diagram above represents the formation of A. a metallic bond B. an electrovalent bond C. a covalent bond D. a coordinate covalent bond Detailed SolutionCovalent bonds involve the sharing of electrons between two atoms |
|
| 50. |
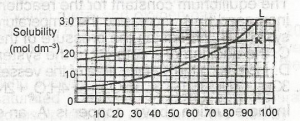 If 1 dm3 of a saturated solution of L at 600C is cooled to 250C, what amount in mole will separate out? A. 0.75 B. 0.25 C. 1.00 D. 0.50 |
A |
| 41. |
The gas that can best be collected by downward displacement of air is A. Chlorine B. Sulphur (IV) oxide C. Ammonia D. Carbon (IV) oxide |
C |
| 42. |
Which of the following metals burn with brick red flame? A. Pb B. Ca C. Na D. Mg |
B |
| 43. |
Which of the following represent hybridization in ethyne? A. Sp2 B. Sp2d C. Sp3 D. Sp |
D |
| 44. |
C12H22O11(s) + H2SO4(aq) 12C(s) + 11H2O(l) + H2SO4(aq) A. A dehydrate agent B. An oxidizing agent C. A reducing agent D. A catalyst |
A |
| 45. |
When sodium reacts with water, the resulting solution is A. Weakly acidic B. Neutral C. Acidic D. Alkaline |
D |
| 46. |
Which of the following gases contains the least number of atoms at s.t.p? A. 1 mole of butane B. 3 mole of ozone C. 4 mole of chlorine D. 7 mole of argon |
A |
| 47. |
 If the gas is cooled, at what temperature will it start to condense? A. 125 oC B. 150oC C. 175oC D. 250oC |
B |
| 48. |
 How long does it take all the solid to melt?` A. 2.5 mins B. 6.0 mins C. 1.0 min D. 3.0 mins |
A |
| 49. |
 The diagram above represents the formation of A. a metallic bond B. an electrovalent bond C. a covalent bond D. a coordinate covalent bond Detailed SolutionCovalent bonds involve the sharing of electrons between two atoms |
|
| 50. |
 If 1 dm3 of a saturated solution of L at 600C is cooled to 250C, what amount in mole will separate out? A. 0.75 B. 0.25 C. 1.00 D. 0.50 |
A |