Year :
1989
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
Mortar is NOT used for under-water construction because A. it hardens by lose of water B. its hardening doe not depend upon evaporation C. it requires concrete to harden D. it will be washed away by the flow of water |
A |
| 32. |
Which of the following is NOT involved in the extraction of metals from their ores? A. reduction with carbon B. reduction with other metals C. reduction by electrolysis D. oxidation with oxidizing agents |
D |
| 33. |
When excess chlorine is mixed with ethene at room temperature, the product is A. 1,2 - dichloroethane B. 1,2 - dichloroethene C. 1,1 - dichloroethane D. 1, 1 - dichloroethene |
A |
| 34. |
Vulcanization of rubber is a process by which A. isoprene units are joined to produce rubber B. rubber latex is coagulated C. sulphur is chemically combined in the rubber D. water is removed from the rubber |
C |
| 35. |
The reaction between ethanoic acid and sodium hydroxide is an example of A. esterification B. neutralization C. hydroxylation D. hydrolysis |
B |
| 36. |
The bond which joins two ethanoic acid molecules in the liquid state is A. a covalent bond B. an ionic bond C. a dative covalent bond D. a hydrogen bond |
D |
| 37. |
The alkaline hydrolysis of fats and oils produces soap and A. propane 1, 1, 3 - triol B. propane - 1,2,3 - triol C. propane - 1,3,3 - triol D. propane - 1, 2, 2 - triol |
B |
| 38. |
The gas responsible for most of the fatal explosions in coal mines is A. butane B. ethene C. ethane D. methane |
D |
| 39. |
Three liquids X, Y and Z containing only hydrogen and carbon were burnt on a spoon. X and Y burnt with sooty flames while Z did not. Y is able to discharge the colour of bromine water whereas X and Z cannot. Which of the liquids would be aromatic in nature? A. X and Z B. Y C. X D. Z Detailed SolutionAromatic compounds burn with a yellow sooty flame and they don't decolorise bromine water, since bromine is not electrophilic enough except in the presence of a strong Lewis catalyst. |
|
| 40. |
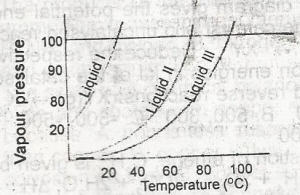 It can be deduced from the vapour pressure curves above that A. liqiud 1 has the highest boiling point B. liquid ll has the highest boiling point C. liquid lll has the highest boiling point D. liquid lll has the lowest boiling point |
C |
| 31. |
Mortar is NOT used for under-water construction because A. it hardens by lose of water B. its hardening doe not depend upon evaporation C. it requires concrete to harden D. it will be washed away by the flow of water |
A |
| 32. |
Which of the following is NOT involved in the extraction of metals from their ores? A. reduction with carbon B. reduction with other metals C. reduction by electrolysis D. oxidation with oxidizing agents |
D |
| 33. |
When excess chlorine is mixed with ethene at room temperature, the product is A. 1,2 - dichloroethane B. 1,2 - dichloroethene C. 1,1 - dichloroethane D. 1, 1 - dichloroethene |
A |
| 34. |
Vulcanization of rubber is a process by which A. isoprene units are joined to produce rubber B. rubber latex is coagulated C. sulphur is chemically combined in the rubber D. water is removed from the rubber |
C |
| 35. |
The reaction between ethanoic acid and sodium hydroxide is an example of A. esterification B. neutralization C. hydroxylation D. hydrolysis |
B |
| 36. |
The bond which joins two ethanoic acid molecules in the liquid state is A. a covalent bond B. an ionic bond C. a dative covalent bond D. a hydrogen bond |
D |
| 37. |
The alkaline hydrolysis of fats and oils produces soap and A. propane 1, 1, 3 - triol B. propane - 1,2,3 - triol C. propane - 1,3,3 - triol D. propane - 1, 2, 2 - triol |
B |
| 38. |
The gas responsible for most of the fatal explosions in coal mines is A. butane B. ethene C. ethane D. methane |
D |
| 39. |
Three liquids X, Y and Z containing only hydrogen and carbon were burnt on a spoon. X and Y burnt with sooty flames while Z did not. Y is able to discharge the colour of bromine water whereas X and Z cannot. Which of the liquids would be aromatic in nature? A. X and Z B. Y C. X D. Z Detailed SolutionAromatic compounds burn with a yellow sooty flame and they don't decolorise bromine water, since bromine is not electrophilic enough except in the presence of a strong Lewis catalyst. |
|
| 40. |
 It can be deduced from the vapour pressure curves above that A. liqiud 1 has the highest boiling point B. liquid ll has the highest boiling point C. liquid lll has the highest boiling point D. liquid lll has the lowest boiling point |
C |