Year :
2003
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 48 of 48 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
The formula for ethyl butanoate is A. C3H7COOC2H5 B. C2H5COOC3H7 C. C4H9COOC2H5 D. C2H5COOC4H9 Detailed SolutionEthyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH₃CH₂CH₂COOCH₂CH₃. |
|
| 42. |
The type of reaction that is peculiar to benzene is? A. addition B. hydrolysis C. polymerization D. substitution Detailed SolutionBenzene undergoes substitution reaction. |
|
| 43. |
Ethanol reacts with excess acidified K2Cr2O to produce A. ethanedioic acid B. ethanal C. ethylethanoate D. ethanoic acid |
D |
| 44. |
A compound contains 40.0% carbon 6.7% hydrogen and 53.3% oxygen. If the molar mass of the compound is 180, find the molecular formula. A. CH2O B. C3H6O3 C. C6H12O6 D. C6H12O3 Detailed SolutionC - 40% ; 40/12 = 3.33H - 6.7% ; 6.7/1 = 6.7 O - 53.3% ; 53.3/16 = 3.33 C = 3.33/3.33 = 1 H = 6.7/3.33 = 2 O = 3.33/3.33 = 1 CH2O (CH2O)n = 180 (12 + 2 + 16)~n = 180 30n = 180 n = 6 = C6H12O6 |
|
| 45. |
The process by which atoms are rearranged into different molecular structure in the petroleum refining process is referred to as A. catalytic cracking B. hydrocracking C. polymerization D. reforming |
D |
| 46. |
Which of the following is found in cotton A. Starch B. Cellulose C. Fat D. Oil |
B |
| 47. |
The principal constituent of natural gas is A. methane B. ethane C. propane D. butane |
A |
| 48. |
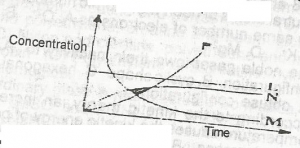 2HCI(ag) + CaCO3(s) → CaCl2(s) + CO 2(g) +H2O(1) From the reaction above, which of the curves in the diagram represents the production of carbon (IV) oxide as dilute HCI is added? A. L B. M C. N D. P |
B |
| 41. |
The formula for ethyl butanoate is A. C3H7COOC2H5 B. C2H5COOC3H7 C. C4H9COOC2H5 D. C2H5COOC4H9 Detailed SolutionEthyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH₃CH₂CH₂COOCH₂CH₃. |
|
| 42. |
The type of reaction that is peculiar to benzene is? A. addition B. hydrolysis C. polymerization D. substitution Detailed SolutionBenzene undergoes substitution reaction. |
|
| 43. |
Ethanol reacts with excess acidified K2Cr2O to produce A. ethanedioic acid B. ethanal C. ethylethanoate D. ethanoic acid |
D |
| 44. |
A compound contains 40.0% carbon 6.7% hydrogen and 53.3% oxygen. If the molar mass of the compound is 180, find the molecular formula. A. CH2O B. C3H6O3 C. C6H12O6 D. C6H12O3 Detailed SolutionC - 40% ; 40/12 = 3.33H - 6.7% ; 6.7/1 = 6.7 O - 53.3% ; 53.3/16 = 3.33 C = 3.33/3.33 = 1 H = 6.7/3.33 = 2 O = 3.33/3.33 = 1 CH2O (CH2O)n = 180 (12 + 2 + 16)~n = 180 30n = 180 n = 6 = C6H12O6 |
| 45. |
The process by which atoms are rearranged into different molecular structure in the petroleum refining process is referred to as A. catalytic cracking B. hydrocracking C. polymerization D. reforming |
D |
| 46. |
Which of the following is found in cotton A. Starch B. Cellulose C. Fat D. Oil |
B |
| 47. |
The principal constituent of natural gas is A. methane B. ethane C. propane D. butane |
A |
| 48. |
 2HCI(ag) + CaCO3(s) → CaCl2(s) + CO 2(g) +H2O(1) From the reaction above, which of the curves in the diagram represents the production of carbon (IV) oxide as dilute HCI is added? A. L B. M C. N D. P |
B |