Year :
1982
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 49 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
Which of the following is NOT a redox reaction? A. 2HNO2 + 2HI → 2H2O + 2NO + I2 B. Zn + H2SO4 → ZnSO4 + H2 C. Ca(HCO3)2 → CaCO3 + H2O + CO2 D. 4FeO + O2 → 2Fe2O3 E. 2Na + Cl2 → 2NaCl Detailed SolutionCa(HCO2) → CaCO3 + H2O + CO2 is not a redox reaction |
|
| 42. |
0.16g of methane when burnt raises the temperature of 100g of water by 40°C. What is the of combustion of methane when if the heat capacity of water is 4.2 Jg-1°C-1? A. 1,160 kJ mol-1 B. 1,180 kJ mol-1 C. 1,560 kJ mol-1 D. 1,600kJ mol-1 E. 1,680 kJ mol-1 Detailed SolutionThe heat of combustion of methane is 1680 KJ mol-1 |
|
| 43. |
Which of the following processes NOT lead to a chemical change? A. Strirring iron in sulphur (IV) acid B. Stirring sodium carbonate in water C. Stirring glucose in concentrated sulphur (IV) acid D. Mixing sulphur (IV) acid with potassium carbonate E. Titrating an acid against base Detailed SolutionStirring Na2CO3 in water does not lead to a chemical change |
|
| 44. |
Brass is an alloy containing copper and? A. zinc B. tin C. aluminium D. silver E. lead Detailed SolutionBrass is an alloy containing copper and zinc |
|
| 45. |
The number of grammes of potassium hydroxide in 250cm3 of one molar solution is? A. 40g B. 52g C. 100g D. 26g E. 14g Detailed Solution1000 cm3 of KOH contains 56 gm of KOH250 cm3 of 1M KOH will contain (250)/(1000) x (56)/(1) = 14gms |
|
| 46. |
60cm3 of hydrogen are sparked with 20cm3 of oxygen at 100°C and 1 atmosphere. The total volume of the residual gases is? A. 60 cm3 B. 10 cm3 C. 40 cm3 D. 30 cm3 E. 70 cm3 |
A |
| 47. |
 The solubility curve of a solid X (molecular mass = 160) is as show above. 1,000 cm3 of a saturated solution of X at 80°C is cooled to 40°C . The weight of X crystalized out would be A. 10 g B. 12 g C. 14 g D. 16 g E. 18 g Detailed SolutionAt 80°C, we have \(1000cm^{3} = 0.4mol/cm^{3}\)40°C = \(0.3mol/cm^{3}\) hence, the loss in concentration = \(0.1mol/cm^{3}\) = \(0.1 \times 160 = 16g\) |
|
| 48. |
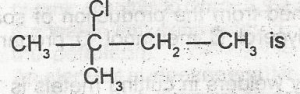 The i.u.p.A.C name for the compound A. 2-chloro-isopentane B. 3-chloro-isopentane C. 2-chloro-2-methylbutane D. 1-chloro-2,2- dimethylpropane E. 2-chloro-2-ethylpropane |
C |
| 49. |
1.00g of mixture of calcium carbonate and calcium oxide liberated 0.33g of carbon dioxide on strong heating. The percentage of calcium oxide in the mixture is? (Ca = 40, C = 12, O = 16) A. 5 B. 15 C. 25 D. 35 E. 50 Detailed SolutionCaCO3 + CaO \(\to\) 2CaO + 2CO244gm of CO3 is liberated by 100gm of CaCO3 0.33gm of CO2 will be liberated by \(\frac{100}{44}\) x 0.33 = 0.75 of CaCO3 Amount of CaO = 1 - 0.75 = 0.25 % of CaO = \(\frac{0.25}{1} \times \frac{100}{1}\) = 25% |
| 41. |
Which of the following is NOT a redox reaction? A. 2HNO2 + 2HI → 2H2O + 2NO + I2 B. Zn + H2SO4 → ZnSO4 + H2 C. Ca(HCO3)2 → CaCO3 + H2O + CO2 D. 4FeO + O2 → 2Fe2O3 E. 2Na + Cl2 → 2NaCl Detailed SolutionCa(HCO2) → CaCO3 + H2O + CO2 is not a redox reaction |
|
| 42. |
0.16g of methane when burnt raises the temperature of 100g of water by 40°C. What is the of combustion of methane when if the heat capacity of water is 4.2 Jg-1°C-1? A. 1,160 kJ mol-1 B. 1,180 kJ mol-1 C. 1,560 kJ mol-1 D. 1,600kJ mol-1 E. 1,680 kJ mol-1 Detailed SolutionThe heat of combustion of methane is 1680 KJ mol-1 |
|
| 43. |
Which of the following processes NOT lead to a chemical change? A. Strirring iron in sulphur (IV) acid B. Stirring sodium carbonate in water C. Stirring glucose in concentrated sulphur (IV) acid D. Mixing sulphur (IV) acid with potassium carbonate E. Titrating an acid against base Detailed SolutionStirring Na2CO3 in water does not lead to a chemical change |
|
| 44. |
Brass is an alloy containing copper and? A. zinc B. tin C. aluminium D. silver E. lead Detailed SolutionBrass is an alloy containing copper and zinc |
|
| 45. |
The number of grammes of potassium hydroxide in 250cm3 of one molar solution is? A. 40g B. 52g C. 100g D. 26g E. 14g Detailed Solution1000 cm3 of KOH contains 56 gm of KOH250 cm3 of 1M KOH will contain (250)/(1000) x (56)/(1) = 14gms |
| 46. |
60cm3 of hydrogen are sparked with 20cm3 of oxygen at 100°C and 1 atmosphere. The total volume of the residual gases is? A. 60 cm3 B. 10 cm3 C. 40 cm3 D. 30 cm3 E. 70 cm3 |
A |
| 47. |
 The solubility curve of a solid X (molecular mass = 160) is as show above. 1,000 cm3 of a saturated solution of X at 80°C is cooled to 40°C . The weight of X crystalized out would be A. 10 g B. 12 g C. 14 g D. 16 g E. 18 g Detailed SolutionAt 80°C, we have \(1000cm^{3} = 0.4mol/cm^{3}\)40°C = \(0.3mol/cm^{3}\) hence, the loss in concentration = \(0.1mol/cm^{3}\) = \(0.1 \times 160 = 16g\) |
|
| 48. |
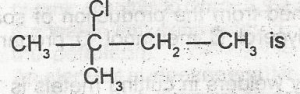 The i.u.p.A.C name for the compound A. 2-chloro-isopentane B. 3-chloro-isopentane C. 2-chloro-2-methylbutane D. 1-chloro-2,2- dimethylpropane E. 2-chloro-2-ethylpropane |
C |
| 49. |
1.00g of mixture of calcium carbonate and calcium oxide liberated 0.33g of carbon dioxide on strong heating. The percentage of calcium oxide in the mixture is? (Ca = 40, C = 12, O = 16) A. 5 B. 15 C. 25 D. 35 E. 50 Detailed SolutionCaCO3 + CaO \(\to\) 2CaO + 2CO244gm of CO3 is liberated by 100gm of CaCO3 0.33gm of CO2 will be liberated by \(\frac{100}{44}\) x 0.33 = 0.75 of CaCO3 Amount of CaO = 1 - 0.75 = 0.25 % of CaO = \(\frac{0.25}{1} \times \frac{100}{1}\) = 25% |