Year :
1986
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
31 - 40 of 47 Questions
| # | Question | Ans |
|---|---|---|
| 31. |
Which of the following substance is a mixture? A. Granulated sugar B. Sea-water C. Sodium chloride D. Iron fillings |
B |
| 32. |
The general formula of an alkyl halide (where X represents the halide) is? A. CnH2n 2X B. CnH2n + 1X C. CnH2n + 2X D. CnH2nX |
B |
| 33. |
Which of the following are made by the process of polymerization? A. Nylon and soap B. Nylon and artificial rubber C. Soap and butane D. Margarine and nylon |
B |
| 34. |
Starch can be converted to ethyl alcohol by? A. distillation B. fermentation C. isomerization D. cracking |
B |
| 35. |
The movement of liquid molecules from the surface of the liquid to the gaseous phase above it is known as? A. Brownian movement B. condensation C. evaporation D. liquefaction |
C |
| 36. |
What mass of a divalent metal M (atomic mass = 40) would react with excess hydrogen gas measured at S.T.P? A. 8.0 g B. 4.0 g C. 0.8 g D. 0.4 g |
D |
| 37. |
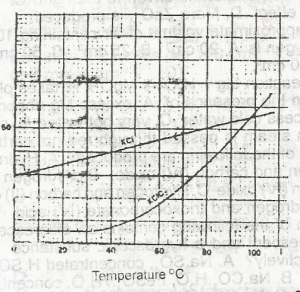 In the solubility curve above, water at 98oC is saturated with KCL and KCIO 3 What is the percentage of KCL impurity in the crystals formed when the solution is cooled to 30 oC? A. 51.5 B. 45.5 C. 34.5 D. 26.5 |
C |
| 38. |
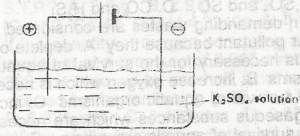 In the electrolysis of aqueous solution of K2SO 4 in the above cell, which species migrate to the anode? A. SO2-OH - B. K + and SO 4 2- C. OH and HO D. H3O + and K |
A |
| 39. |
 The tabulated results below were obtained by titration 10.0 cm3 of water with soup. The titration was repeated with the same sample of water after boiling. The ratio of permanent to temporary hardness is A. 1.5 B. 1.4 C. 4.1 D. 5.1 Detailed SolutionThe ratio of permanent to temporary hardness will be 20:(20 - 15)= 20:5 = 4:1 |
|
| 40. |
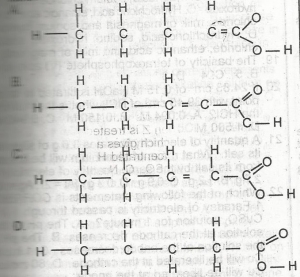 Which is the structural formula for pent-2-enoic acid? A. A B. B C. C D. D |
C |
| 31. |
Which of the following substance is a mixture? A. Granulated sugar B. Sea-water C. Sodium chloride D. Iron fillings |
B |
| 32. |
The general formula of an alkyl halide (where X represents the halide) is? A. CnH2n 2X B. CnH2n + 1X C. CnH2n + 2X D. CnH2nX |
B |
| 33. |
Which of the following are made by the process of polymerization? A. Nylon and soap B. Nylon and artificial rubber C. Soap and butane D. Margarine and nylon |
B |
| 34. |
Starch can be converted to ethyl alcohol by? A. distillation B. fermentation C. isomerization D. cracking |
B |
| 35. |
The movement of liquid molecules from the surface of the liquid to the gaseous phase above it is known as? A. Brownian movement B. condensation C. evaporation D. liquefaction |
C |
| 36. |
What mass of a divalent metal M (atomic mass = 40) would react with excess hydrogen gas measured at S.T.P? A. 8.0 g B. 4.0 g C. 0.8 g D. 0.4 g |
D |
| 37. |
 In the solubility curve above, water at 98oC is saturated with KCL and KCIO 3 What is the percentage of KCL impurity in the crystals formed when the solution is cooled to 30 oC? A. 51.5 B. 45.5 C. 34.5 D. 26.5 |
C |
| 38. |
 In the electrolysis of aqueous solution of K2SO 4 in the above cell, which species migrate to the anode? A. SO2-OH - B. K + and SO 4 2- C. OH and HO D. H3O + and K |
A |
| 39. |
 The tabulated results below were obtained by titration 10.0 cm3 of water with soup. The titration was repeated with the same sample of water after boiling. The ratio of permanent to temporary hardness is A. 1.5 B. 1.4 C. 4.1 D. 5.1 Detailed SolutionThe ratio of permanent to temporary hardness will be 20:(20 - 15)= 20:5 = 4:1 |
|
| 40. |
 Which is the structural formula for pent-2-enoic acid? A. A B. B C. C D. D |
C |