Year :
2004
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 50 of 50 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
An isomer of C5H12 is A. Butane B. 2 – methylbutane C. 2 – methylpropane D. 2 – ethylbutane Detailed SolutionIsomers of Pentane include : 2 methyl-butane and 2-Dimethylpropane.So Option B is most viable. |
|
| 42. |
A characteristic of the alkane family is A. Addition reaction B. Elimination reaction C. Substitution reaction D. Neutralization reaction |
C |
| 43. |
Which of these reagents can confirm the presence of a triple bond? A. Bromine water B. Acidified KMnO4 C. Copper (I) chloride D. Bromine gas |
C |
| 44. |
Vulcanization involves the removal of A. A monomer B. The single bond C. The double bond D. A polymer |
C |
| 45. |
CH3COOH(g) → H4(g) + CO2(g) A. Carboxylation B. Decarboxylation C. Acidification D. Esterification |
B |
| 46. |
C2H5OH(aq) → Y A. CH4 B. C2H5COOH C. C2H4 D. CH3OCH3 |
C |
| 47. |
Alkanol + Alkanoic ↔ Ester + Water A. Hydrolysis B. Saponification C. Hydration D. Fermentation |
A |
| 48. |
In the production of soap, concentrated sodium chloride solution is added to A. Increase the solubility of soap B. Decrease the solubility of the soap C. Saponify the soap D. Emulsify the soap |
B |
| 49. |
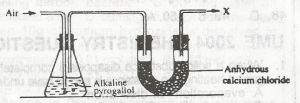 In the experiment above, X is a mixture of nitrogen, carbon (IV) oxide and A. water B. impurities C. inert gas D. oxygen |
C |
| 50. |
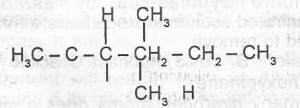 The IUPAC nomenclature of the compound above is A. 2-ethylpentane B. 3,4-dimethylhexane C. 2,3-dimethylhexane D. 2-ethylhexane |
B |
| 41. |
An isomer of C5H12 is A. Butane B. 2 – methylbutane C. 2 – methylpropane D. 2 – ethylbutane Detailed SolutionIsomers of Pentane include : 2 methyl-butane and 2-Dimethylpropane.So Option B is most viable. |
|
| 42. |
A characteristic of the alkane family is A. Addition reaction B. Elimination reaction C. Substitution reaction D. Neutralization reaction |
C |
| 43. |
Which of these reagents can confirm the presence of a triple bond? A. Bromine water B. Acidified KMnO4 C. Copper (I) chloride D. Bromine gas |
C |
| 44. |
Vulcanization involves the removal of A. A monomer B. The single bond C. The double bond D. A polymer |
C |
| 45. |
CH3COOH(g) → H4(g) + CO2(g) A. Carboxylation B. Decarboxylation C. Acidification D. Esterification |
B |
| 46. |
C2H5OH(aq) → Y A. CH4 B. C2H5COOH C. C2H4 D. CH3OCH3 |
C |
| 47. |
Alkanol + Alkanoic ↔ Ester + Water A. Hydrolysis B. Saponification C. Hydration D. Fermentation |
A |
| 48. |
In the production of soap, concentrated sodium chloride solution is added to A. Increase the solubility of soap B. Decrease the solubility of the soap C. Saponify the soap D. Emulsify the soap |
B |
| 49. |
 In the experiment above, X is a mixture of nitrogen, carbon (IV) oxide and A. water B. impurities C. inert gas D. oxygen |
C |
| 50. |
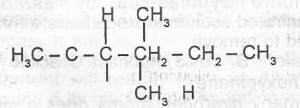 The IUPAC nomenclature of the compound above is A. 2-ethylpentane B. 3,4-dimethylhexane C. 2,3-dimethylhexane D. 2-ethylhexane |
B |