Year :
1991
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 49 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
A certain liquid has a high boiling point. It is viscous, non-toxic, miscible with water being very hygroscopic. This liquid is most likely to be? A. CH3CH2CH2HO B. CH3CHOHCH3 C. CH3CH2CHOHCH3 D. CH2OHCHOHCH2OH |
D |
| 42. |
CH3-CH-CH3 A. 1-chloro-2-methylbutane B. 1-chloro-2-methylpropane C. 2-chloromethylpropane D. 1-chloro-2, 2-dimethylethane |
B |
| 43. |
Which of the following statements is TRUE of the complete hydrolysis of a glycerine by sodium hygroxide? A. 3 moles ofNaOH are required for each mole of glyceride B. 3 moles of glycerol are produced C. Only one mole of soap is formed D. concentrated H2SO4 is essential for the completion of the reaction |
A |
| 44. |
Which of the following are the products of the reaction between CH3COOH and CI2 in sunlight? A. CICH2COOH + HCI B. CH3COCI + HOCI C. CH3COOCI + HCI D. CH3COCI + HO2 |
A |
| 45. |
Which of these is an acid salt? A. K2SO4-.AI2(SO4)3.24H2O B. CuCO3.Cu(OH)2 C. NaHS D. CaOCI2- |
C |
| 46. |
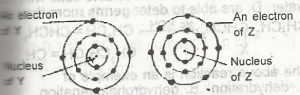 The electrons of two atoms Y and Z are arranged in shells as shown above. The bond formed between the atoms of Y and Z is A. ionic B. covalent C. dative D. metallic Detailed SolutionElement Y is SiliconElement Z is Chlorine Silicon reacts with chlorine to form the covalent compound SiCl4 |
|
| 47. |
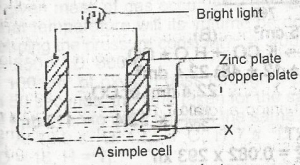 50 cm3 of sulphur (lV) oxide, 800 cm3 of carbon (IV) oxide will respectively saturate 1.0cm3 of water at 15oC. Which of the following is suitable for demonstrating the fountain experiment? A. Sulphur (IV) oxide and hydrogen chloride. B. Carbon (IV) oxide and ammonia C. Ammonia and hydrogen chloride D. Carbon (IV) oxide and Sulphur (IV) oxide |
C |
| 48. |
 The data in the table above shows the rate of reaction of nitrogen (ll) oxide with chlorine at 25oC It can be concluded that doubling the initial concentration of NO increase the rate of reaction by a factor of A. two B. three C. four D. five Detailed Solution3.0 x 10-5 x 4 = 1.2 x 10-4.: The rate increase by a factor of 4 |
|
| 49. |
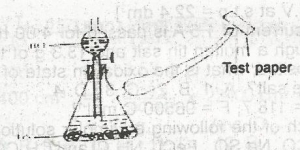 The appropriate test paper to use in the above experiment is moist A. litmus psper B. potassium heptaoxodichromate (IV) paper C. lead (ll) trioxonitrate (V) paper D. universal indicator paper |
C |
| 41. |
A certain liquid has a high boiling point. It is viscous, non-toxic, miscible with water being very hygroscopic. This liquid is most likely to be? A. CH3CH2CH2HO B. CH3CHOHCH3 C. CH3CH2CHOHCH3 D. CH2OHCHOHCH2OH |
D |
| 42. |
CH3-CH-CH3 A. 1-chloro-2-methylbutane B. 1-chloro-2-methylpropane C. 2-chloromethylpropane D. 1-chloro-2, 2-dimethylethane |
B |
| 43. |
Which of the following statements is TRUE of the complete hydrolysis of a glycerine by sodium hygroxide? A. 3 moles ofNaOH are required for each mole of glyceride B. 3 moles of glycerol are produced C. Only one mole of soap is formed D. concentrated H2SO4 is essential for the completion of the reaction |
A |
| 44. |
Which of the following are the products of the reaction between CH3COOH and CI2 in sunlight? A. CICH2COOH + HCI B. CH3COCI + HOCI C. CH3COOCI + HCI D. CH3COCI + HO2 |
A |
| 45. |
Which of these is an acid salt? A. K2SO4-.AI2(SO4)3.24H2O B. CuCO3.Cu(OH)2 C. NaHS D. CaOCI2- |
C |
| 46. |
 The electrons of two atoms Y and Z are arranged in shells as shown above. The bond formed between the atoms of Y and Z is A. ionic B. covalent C. dative D. metallic Detailed SolutionElement Y is SiliconElement Z is Chlorine Silicon reacts with chlorine to form the covalent compound SiCl4 |
|
| 47. |
 50 cm3 of sulphur (lV) oxide, 800 cm3 of carbon (IV) oxide will respectively saturate 1.0cm3 of water at 15oC. Which of the following is suitable for demonstrating the fountain experiment? A. Sulphur (IV) oxide and hydrogen chloride. B. Carbon (IV) oxide and ammonia C. Ammonia and hydrogen chloride D. Carbon (IV) oxide and Sulphur (IV) oxide |
C |
| 48. |
 The data in the table above shows the rate of reaction of nitrogen (ll) oxide with chlorine at 25oC It can be concluded that doubling the initial concentration of NO increase the rate of reaction by a factor of A. two B. three C. four D. five Detailed Solution3.0 x 10-5 x 4 = 1.2 x 10-4.: The rate increase by a factor of 4 |
|
| 49. |
 The appropriate test paper to use in the above experiment is moist A. litmus psper B. potassium heptaoxodichromate (IV) paper C. lead (ll) trioxonitrate (V) paper D. universal indicator paper |
C |