Year :
2006
Title :
Chemistry
Exam :
JAMB Exam
Paper 1 | Objectives
41 - 49 of 49 Questions
| # | Question | Ans |
|---|---|---|
| 41. |
In the electrolysis of CuSO4(g) using platinum electrodes, the reaction at the anode is A. 4H+ + 4e- → 2H2 B. 4OH- - 4e- → 2H2O + O2 C. 2OH - 2e- → 2OH D. 2OH- + 2OH- → 2H2O + O2 |
B |
| 42. |
Na2CO3(g) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g) A. 1.000 mole B. 0.100 mole C. 0.010 mole` D. 0.111 mole Detailed Solution\(\frac{C_A V_A}{C_BV_B} = \frac{a}{b}\)\(\frac{0.05 \times 10}{C_B \times 25} = \frac{2}{1}\) 50CB = 0.5 CB = \(\frac{0.5}{50} = \frac{1}{100}\) = 0.01 |
|
| 43. |
If the heat of combustion of hydrogen is -285.8kJ, A. +571.6 kJ B. -571.6 kJ C. -285.8 kJ D. +285.8 kJ |
C |
| 44. |
5SO2(g) + 2KMnO4(aq) + 2H2O(l) → K2SO4 + 2MnSO4(aq) + 2H2SO4(aq) A. purple solution B. purple precipitate C. colourless precipitate D. colourless solution |
D |
| 45. |
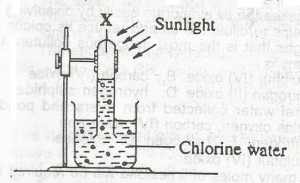 In the diagram above, gas X is A. hydrogen B. chlorine C. oxygen D. hydrogen chloride |
C |
| 46. |
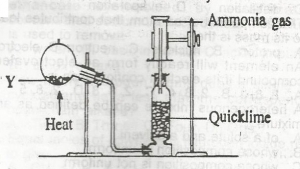 In the diagram above, mixture Y is A. NH4NO3(s) + CaSO4(s),/sub> B. NH4CL(s) + NaNO2(s) C. NH4NO2(s) + Na2SO4(s) D. NH4CL(s) + Ca(OH)2(s) |
D |
| 47. |
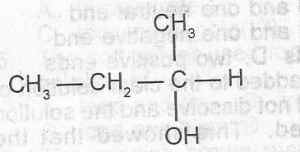 The oxidation of the compound above produces A. 2-butanone B. 2-butanal C. 3-butanal D. 3-butanone |
A |
| 48. |
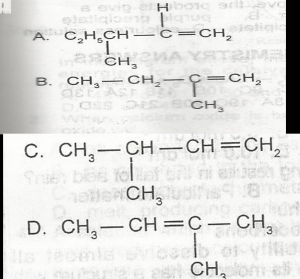 2-methylbut-2-ene has the structure A. A B. B C. C D. D |
D |
| 49. |
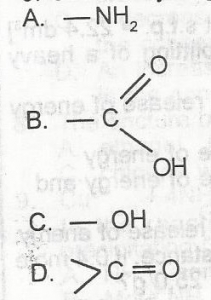 Which of the following functional groups will give gas bubbles when treated with a saturated solution of sodium hydrogen trioxocarbonate (IV)? A. A B. B C. C D. D |
B |
| 41. |
In the electrolysis of CuSO4(g) using platinum electrodes, the reaction at the anode is A. 4H+ + 4e- → 2H2 B. 4OH- - 4e- → 2H2O + O2 C. 2OH - 2e- → 2OH D. 2OH- + 2OH- → 2H2O + O2 |
B |
| 42. |
Na2CO3(g) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g) A. 1.000 mole B. 0.100 mole C. 0.010 mole` D. 0.111 mole Detailed Solution\(\frac{C_A V_A}{C_BV_B} = \frac{a}{b}\)\(\frac{0.05 \times 10}{C_B \times 25} = \frac{2}{1}\) 50CB = 0.5 CB = \(\frac{0.5}{50} = \frac{1}{100}\) = 0.01 |
|
| 43. |
If the heat of combustion of hydrogen is -285.8kJ, A. +571.6 kJ B. -571.6 kJ C. -285.8 kJ D. +285.8 kJ |
C |
| 44. |
5SO2(g) + 2KMnO4(aq) + 2H2O(l) → K2SO4 + 2MnSO4(aq) + 2H2SO4(aq) A. purple solution B. purple precipitate C. colourless precipitate D. colourless solution |
D |
| 45. |
 In the diagram above, gas X is A. hydrogen B. chlorine C. oxygen D. hydrogen chloride |
C |
| 46. |
 In the diagram above, mixture Y is A. NH4NO3(s) + CaSO4(s),/sub> B. NH4CL(s) + NaNO2(s) C. NH4NO2(s) + Na2SO4(s) D. NH4CL(s) + Ca(OH)2(s) |
D |
| 47. |
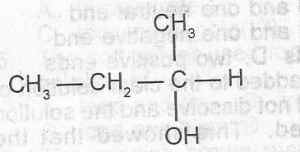 The oxidation of the compound above produces A. 2-butanone B. 2-butanal C. 3-butanal D. 3-butanone |
A |
| 48. |
 2-methylbut-2-ene has the structure A. A B. B C. C D. D |
D |
| 49. |
 Which of the following functional groups will give gas bubbles when treated with a saturated solution of sodium hydrogen trioxocarbonate (IV)? A. A B. B C. C D. D |
B |