
Chemistry
Paper 1 | Objectives | 40 Questions
JAMB Exam
Year: 2020
Level: SHS
Time:
Type: Question Paper
Answers provided
FREE
No description provided
Feedbacks
This paper is yet to be rated

Paper 1 | Objectives | 40 Questions
JAMB Exam
Year: 2020
Level: SHS
Time:
Type: Question Paper
Answers provided
No description provided
This paper is yet to be rated
Past questions are effective for revisions for all tests including WAEC, BECE, SAT, TOEFL, GCSE, IELTS
There's no secret to passing WAEC or BECE apart from the merit of hard work and adequate preparation
The goal of mocks tests is to create a bench-marking tool to help students assess their performances.
| # | Question | Ans |
|---|---|---|
| 1. |
The electronic configuration of an element is 1S\(^2\) 2S\(^2\) 2P\(^6\) 3S\(^2\) 3P\(^3\). How many unpaired electrons are there in the element? A. 5 B. 4 C. 3 D. 2
Show Content
Detailed SolutionOnce you figure out the electron configuration, you fill up the corresponding orbitals with electrons, any left with one is considered unpaired. Since 1s can only hold 2 electrons, and P has 15, that's obviously filled and has no unpaired electrons. The same is for 2s which holds 2, 2p which holds 6, 3s which holds 2.However 3p can hold 6 electrons and in order for that to be filled up you would need to have an element of 18 electrons. So you fill up as much as you can in 3p by first adding 1 electron to each energy level. 3p has 3 energy levels and there are only 3 electrons left to distribute, so each of those energy levels only gets 1, because you have to fill them all with one before you can start addin |
|
| 2. |
Which of the following can be obtained by fractional distillation? A. Nitrogen from liquid air B. Sodium Chloride from sea water C. Iodine from solution of Iodine in carbon tetrachloride D. Sulphur from the solution of sulphur in carbon disulphide
Show Content
Detailed SolutionFractional distillation is used to separate mixtures into its component parts or fractions with the difference in boiling points between successive fractions must be more than 10°C. An example is the liquid nitrogen and oxygen are then separated by fractional distillation .There is an explanation video available below. |
|
| 3. |
Duralumin consists of aluminum, copper? A. Zinc and Gold B. Lead and Manganese C. Nickel and Silver D. Manganese and Magnesium
Show Content
Detailed SolutionDuralumin is an alloy made up of 90% aluminum, 4% copper, 0.51% magnesium, and less than 1% manganese.There is an explanation video available below. |
|
| 4. |
An example of a polysaccharide is? A. Dextrose B. Mannose C. Glucose D. Starch
Show Content
Detailed SolutionPolysaccharides are long chains of monosaccharides linked by glycosidic bonds. Three important polysaccharides, starch, glycogen, and cellulose, are composed of glucose.There is an explanation video available below. |
|
| 5. |
8g of CH\(_4\) occupies 11.2 at S.T.P. What volume would 22g of CH\(_3\)CH\(_2\)CH\(_3\) occupy under the same condition? A. 3.7dm\(^3\) B. 11.2dm\(^3\) C. 22.4dm\(^3\) D. 33dm\(^3\)
Show Content
Detailed SolutionMolar mass of methane = 16g/molAt S.T.P. its 8g occupies 11.2 so 16g occupies 22.4 dm\(^3\) Molar mass of propane = 44g/mol\(^-1\) occupies 22.4dm\(^3\) at S.T.P so 22g will occupy 11.2dm\(^3\) There is an explanation video available below. |
|
| 6. |
The best treatment for a student who accidentally poured conc tetraoxosulphate(vi) on his skin in the laboratory is to wash his skin with? A. with cool running water B. sodium hydroxide solution C. iodine solution D. sodium trioxonitrate(v) solution |
|
| 7. |
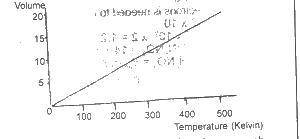 Which of the gas laws does this graph illustrate? A. Boyle B. Charles C. Gay-Lussac D. Graham
Show Content
Detailed SolutionCharles' law describes the effect of temperature changes on the volume of a given mass of gas at a constant pressure.There is an explanation video available below. |
|
| 8. |
What are the possible oxidation numbers of an element if its atomic number is 17? A. -1 and 7 B. -1 and 6 C. -3 and 5 D. -2 and 6
Show Content
Detailed SolutionThe element with atomic number 17 is Chlorine with a valency of -1, that is it needs to receive an electron or possibly lose seven electrons to obey the octet rule.There is an explanation video available below. |
Preview displays only 8 out of the 40 Questions